transcript
transcript
White House Will Send Vaccinations Directly to Pharmacies
The Biden administration said on Tuesday that it would allocate a minimum of 10.5 million doses of coronavirus vaccines to states in the next three weeks, and would also send some doses directly to retail pharmacies.
-
Last week, we took steps to increase the amount of vaccine going to states, tribes and territories by 16 percent. Today, we are further increasing that weekly allocation by an additional 5 percent. So for the next three weeks, we will provide a minimum of 10.5 million in total doses per week across all jurisdictions. That means we have increased supply by more than 20 percent since we took office. To be clear, this is on top of the 10.5 million doses we will ship this week. I also want to be clear that we are delivering on our commitment to provide states with three weeks forward visibility into supply. This is critical, and we’ve heard strong feedback from state and local leaders that this is helping give them what they need to plan and get vaccines administered more quickly. Pharmacies are readily accessible in most communities, with most Americans living within five miles of a pharmacy. That’s why we’re pleased to announce our first phase of the federal retail pharmacy program for Covid-19 vaccinations. This is a key component of President Biden’s national strategy, offering vaccinations in America’s pharmacies. Starting on Feb. 11, the federal government will deliver vaccines directly to select pharmacies across the country. This will provide more sites for people to get vaccinated in their communities, and it’s an important component to delivering vaccines equitably.
The federal government will send one million vaccine doses to about 6,500 retail pharmacies on Feb. 11, the beginning of a federal program that will deliver vaccines directly to as many as 40,000 drugstores and grocery stores, Jeffrey D. Zients, the White House’s Covid-19 response coordinator, announced on Tuesday.
The program’s launch will mark the start of a new stage in the massive vaccination campaign in the United States, gradually shifting a drive which so far has been centered in health care facilities and massive sites like stadiums to smaller, more local settings that will play a crucial role in vaccinating the general public.
While some states in recent weeks have begun using a limited number of retail pharmacies to give out some of their vaccine doses, the federal program launching next week will not cut into the doses allocated to states — and over time, it will greatly expand the number of sites where eligible people can get vaccines.
Still, the launch of the program will be slow at first — many pharmacies will not have vaccines at all or will have very limited supply, Mr. Zients said — and it will not expand the still limited number of high-risk groups that states have deemed to be eligible to receive vaccines. Eligible patients should check pharmacy websites for availability, Mr. Zients said.
Mr. Zients said that the federal government would allocate vaccines under the pharmacy program based on the population of each state and jurisdiction, as it has been doing for vaccines distributed via other channels.
The Centers for Disease Control and Prevention is also working with states to choose pharmacies in areas that are “harder to reach” and close to people at higher risk of severe Covid-19, Mr. Zients said. He added that the agency would monitor the program to make sure pharmacies are distributing doses efficiently and fairly.
Dozens of national and regional chains are participating, though not all of them will be involved in the first phase of distribution. Among them are CVS, Walgreens, Walmart, Rite Aid, Kroger, Publix, Meijer, Costco, Jewel-Osco and Safeway.
CVS said on Tuesday that it would begin offering vaccines on Feb. 11 in 11 states under the federal retail pharmacy program. Walgreens said on Tuesday that it would do the same on Feb. 12 in 15 states and major metropolitan areas. Both chains had already been giving out shots in long-term care facilities and retail pharmacies in some states.
Walmart said last week that it has more than 5,000 stores across its flagship brand and Sam’s Club locations that “are operationally and clinically ready” to give out vaccines.
The Trump administration first announced the retail pharmacy program last fall.

It did not take long for Keith Reed, a deputy health commissioner in Oklahoma, to spot a big logistical problem with the state’s vaccination rollout. Week after week, Oklahoma was allocating thousands of precious doses to a federal program for nursing home patients that was not using them all. In fact, tens of thousands of doses were sitting untouched in freezers.
So his department called an audible. It decided to stop allocating any more of Oklahoma’s vaccine supply to the federal program, a partnership with private pharmacies like CVS and Walgreens that is meant to immunize residents of long-term care facilities. Instead, they would go to distribution channels that would get them into people’s arms faster.
A number of states have made similar moves to shift supplies away from the federal effort, known as the Pharmacy Partnership for Long-Term Care Program, a telling example of how chaotic the inoculation effort in the U.S. has been so far. Some of the other states include Minnesota, Maine, Michigan, Missouri and Ohio.
Mr. Reed said the Oklahoma move would do no harm: Walgreens and CVS have assured him, he said, that all nursing home residents in the state who needed — and wanted — to be vaccinated would have the first of their two shots by the end of the week.
The federal program used a formula that turned out to significantly overestimate how many shots would be needed for long-term care facilities like nursing homes, whose residents are particularly vulnerable to the coronavirus. And another problem arose: A considerable number of residents and, especially, workers at the facilities are turning down the chance to be vaccinated.
A study released Monday by the Centers for Disease Control and Prevention found that in the first month of the program, 77.8 percent of residents and 37.5 percent of workers received the vaccine at the average long-term care facility. The study says the true rate for workers may be higher because some may have been vaccinated in other settings. But, even so, federal officials are particularly concerned about how many workers are refusing inoculation, and have been stepping up efforts to change their minds.
Mr. Reed said the doses Oklahoma was taking away from the federal program will be going to thousands of Oklahomans who are 65 or older and do not live in nursing homes.
“Our goal is to move vaccine from freezers to somebody’s arm within seven days of receiving it,” Mr. Reed said in an interview last week. “We just had a hard time with that amount of vaccine off-limits to us that was set aside for this program, when we could be using that vaccine to go straight to Oklahomans.”
Advocates for nursing home residents are watching closely for any sign that the moves will impede their vaccinations.
“If we find that older adults are not getting the vaccines that they need, then that’s concerning to us,” said Lisa Sanders, a spokeswoman for LeadingAge, which represents more than 5,000 nonprofit aging services providers.

A single dose of the vaccine developed by the University of Oxford and AstraZeneca provided strong protection against Covid-19 in clinical trials when its second shots were delayed by at least three months, researchers reported on Tuesday.
The encouraging results, detailed by Oxford and AstraZeneca researchers in a manuscript that has not been peer-reviewed, lend support to the strategy deployed by Britain and other countries to prioritize providing as many first doses of vaccines as possible without worrying that people will get their second doses later than initially planned.
The latest data do not have bearing on the debate over whether to further space out the doses of the two vaccines authorized in the United States, those from Pfizer-BioNTech and Moderna, since the data on AstraZeneca’s candidate cannot be generalized to other vaccines.
Some scientists have called on the United States to follow the lead of Britain and other countries that have opted to delay the second doses of vaccines by up to 12 weeks. But U.S. federal officials have resisted, saying such a move would not be supported by the data from clinical trials of the two vaccines currently available across the nation. Tuesday’s results could amplify pressure on U.S. health officials to delay second doses of AstraZeneca’s vaccine, though it has not yet been authorized by the country.
The Oxford and AstraZeneca researchers found that a single dose of the vaccine was 76 percent effective at preventing Covid-19. The data measured the three months after the first shot was given, not including an initial three-week period needed for protection to take effect.
The vaccine appeared more effective when the interval between the two shots was longer than the originally intended four-week gap. Among clinical trial participants who got two standard-strength doses at least three months apart, the vaccine was 82 percent effective, compared to 55 percent effective when the doses were given less than six weeks apart.
A vaccination strategy that spaces out doses by three months “may be the optimal for rollout of a pandemic vaccine when supplies are limited in the short term,” the researchers wrote.
Tuesday’s results build on data released late last year, which found that the vaccine was 62 percent effective when given as two standard-strength doses. In those initial findings, the vaccine’s efficacy was much higher, at 90 percent, when the first dose of the vaccine was given at half-strength.
Oxford and AstraZeneca researchers initially attributed the different levels of effectiveness to the lower strength of the initial dose. But they gradually reached a different conclusion: the amount of time between doses was the more likely explanation.
The results on Tuesday were not entirely positive. The researchers also reported that a single dose of the vaccine did not protect against the virus spreading without symptoms. However, overall cases of any positive test for the coronavirus, whether symptomatic or not, were reduced by 67 percent with a single dose. The researchers said that pointed to “the potential for a substantial reduction in transmission.”
In the United States, the Food and Drug Administration is waiting on data from a clinical trial that enrolled about 30,000 participants, mostly Americans. Results from that study are expected later this month.
The study is expected to arm AstraZeneca with enough safety data to allow it by around early March to seek authorization to provide the vaccine for emergency use.
The United States has agreed to buy 300 million doses of AstraZeneca’s vaccine, but neither the company nor the federal government has said when and in what quantities those doses will be available after the vaccine is approved.
A vaccine developed in Russia, Sputnik V, has been shown to have 91.6 percent efficacy against the coronavirus, according to an analysis published in the medical journal The Lancet on Tuesday.
The peer-reviewed results, based on a clinical trial conducted on nearly 22,000 people, showed that the Sputnik V vaccine appeared to be safe and did not cause serious side effects, according to The Lancet, an early vindication for Russia, which faced international skepticism after the vaccine was approved without the release of data regarding clinical trials.
The results bring the number of vaccines whose efficacy is higher than 90 percent to three, and will leave Russia well positioned to deliver a cheap vaccine at home and abroad — two shots of the Sputnik V vaccine are necessary, each costing $10. The Sputnik V shots also do not need the deep cold storage that can make some other vaccines logistically challenging to use widely.
The study comes after the vaccine’s developer, the Gamaleya Research Institute, which is part of the Russian Health Ministry, announced in December that the vaccine showed 91.4 percent efficacy.
When a Russian health care regulator approved the vaccine in August, becoming the first in the world to do so even though the shots had yet to complete clinical trials, experts raised concerns that the authorities were trying to hastily approve a vaccine without due safeguards.
But researchers at the Gamaleya Institute had gone ahead months earlier: The head of the team that developed the Sputnik V vaccine, Denis Logunov, along with some colleagues, administered the vaccine to themselves as early as April, according to a New Yorker investigation.
Ian Jones of the University of Reading and Polly Roy of the London School of Hygiene and Tropical Medicine, who are both virology professors, wrote in The Lancet, “The development of the Sputnik V vaccine has been criticized for unseemly haste, corner cutting, and an absence of transparency.”
“But,” they added, “the outcome reported here is clear and the scientific principle of vaccination is demonstrated.”
Russia began its vaccination campaign in December, and around 50 countries have pre-ordered the vaccine. Use of the Sputnik V vaccine in more than a dozen countries, including Algeria, Hungary, Iran and Venezuela, is set to begin this week.
Mexico, which has pre-ordered more than 7 million doses of the Sputnik V vaccine, has approved it for emergency use, Hugo López-Gatell, the deputy health minister who is leading the nation’s coronavirus response, said at a news conference on Tuesday evening.
In December, the Gamaleya Institute announced that it had partnered with the drug maker AstraZeneca to try to combine their vaccines and see if the mixture could increase efficacy.

Gov. Andrew M. Cuomo of New York said on Tuesday that local governments could begin to provide coronavirus vaccines to restaurant workers, just one day after he dismissed a debate over expanding vaccine eligibility as “a cheap, insincere discussion.”
Taxi drivers and residents at facilities for the developmentally disabled can also be vaccinated, he said.
Mr. Cuomo linked the changes to an increase in the vaccine doses allocated to New York State by the federal government. On Tuesday, the Biden administration said it would bump the supply given to states by 5 percent, which resulted from an expected increase in manufacturing.
“Now there’s additional flexibility,” Mr. Cuomo said. “And I’m leaving it up to the local governments to make a determination of what fits their situation best.”
The debate over whether restaurant workers should be eligible for the vaccine was kicked off last week, when Mr. Cuomo said he would allow restaurants to New York City to resume indoor dining on Feb. 14.
The governor barred indoor dining in the city in December among concerns over a second wave of the coronavirus. At the time, the Centers for Disease Control and Prevention had released recommendations that described eating at indoor restaurants as a “particularly high-risk” activity, and state contact tracing data suggested that restaurants and bars were the fifth main source of new infections in New York.
Still, while hospitalizations and the seven-day average positive test rate have been trending downward in recent weeks, both remain higher than they were when Mr. Cuomo closed the restaurants. New York City is at an extremely high risk level for coronavirus, according to an assessment by The New York Times and public health experts.
With indoor dining set to restart just as more infectious variants are spreading across the country, many were concerned that restaurant workers returning to their jobs would be putting themselves at increased risk.
On Monday, Mr. Cuomo dismissed the suggestion of expanding eligibility, saying the state did not have the vaccine supply necessary to loosen its criteria and that calling for it to do so was a “cheap, insincere discussion.”
“Yes, I would like to see restaurant workers eligible,” Mr. Cuomo said. “It makes total sense. But what does eligibility mean when you don’t have the supply necessary?”
On Tuesday morning, Mayor Bill de Blasio of New York City said that he believed that restaurant workers should be eligible to receive the vaccine immediately.
“Restaurant workers now are going to be in enclosed places with people eating and drinking,” Mr. de Blasio said. “Every doctor on this line or anyplace else will say that’s an area of concern.”
The upstart drugmaker Moderna is asking U.S. regulators to allow it to increase the amount of coronavirus vaccine put into each vial by as much as 50 percent, arguing that it can speed vaccines to patients by clearing away a simple manufacturing bottleneck: Getting medicine into bottles.
The Food and Drug Administration could decide within a few weeks how much more vaccine Moderna, the developer of one of the two federally authorized Covid-19 vaccines, can put into its vials. Moderna says it can raise the number of doses per vial from 10 to as much as 15.
The company has already been ramping up production of its vaccine, only to find a bottleneck in the bottling, capping and labeling process. With F.D.A. approval, more doses could start going into each bottle quickly, a welcome boost to the campaign to curb a pandemic that has killed more than 440,000 people in the United States alone. In a statement late Monday, Ray Jordan, a Moderna spokesman, said the constraint on dosage per vial was limiting Moderna’s output.
The Moderna proposal is part of a broader push by the Biden administration to speed vaccine distribution, including by clearing away obstacles in the “fill and finish” phase of manufacturing. Although the nuts-and-bolts stage receives less attention than vaccine development, it has been identified for years as a constraint on vaccine production.
On Tuesday, Jeffrey D. Zients, the White House’s Covid-19 response coordinator, said that the federal government would allocate a minimum of 10.5 million doses of coronavirus vaccines to states for the next three weeks, a bump of five percent resulting from an expected increase in manufacturing.
At a White House news conference, Mr. Zients framed the increase in doses as an accomplishment of the Biden administration, saying that “we have increased supply by more than 20 percent since we took office.” But the uptick in production has long been expected as the companies that make two federally authorized vaccines, one from Pfizer and BioNTech and the other from Moderna, have scaled up their efforts. Last week, the companies increased their supply to the U.S. by 16 percent.
Governors were informed of the increase on a call Tuesday morning so that they would have more time to plan for vaccinations, Mr. Zients said, with at three weeks of notice for new allocation numbers — a cornerstone of a new effort by the Biden administration to improve a distribution system mired in uncertainty and confusion over limited supply and unused doses.
“That allows them to plan accordingly and know what staffing to have,” he said. “I think historically, there had been fluctuation. We are very attuned into not having that fluctuation.”
Moderna has discussed the possible change of the number of doses in vials with the F.D.A. but has not yet submitted manufacturing data to support it, people familiar with the discussions said. Federal regulators may be receptive to the idea of more doses in each vial, but could balk at the notion of a 50 percent increase.
The industry standard has long been 10 doses per vial, and federal regulators may be concerned that the extra punctures by needles of the rubber covering of the vial and the time required to extract more doses could increase the risk of contaminating the vaccine with bacteria. Moderna’s proposal to the F.D.A. for the dose increase was first reported by CNBC.
Packing more vaccine into each Moderna vial is one of a number of options White House and health officials are exploring as they push to expand production before the spring, when officials are expecting a renewed surge of infections from emerging variants of the virus.
The maker of the other federally approved vaccine, Pfizer, is unable to increase the amount of vaccine in its vials because its manufacturing is geared toward a particular size of vial that can hold only about six doses. But Moderna’s vial is big enough to hold more than the 10 doses now allowed.
Asked about Moderna’s proposal, a White House spokesman on Monday said that “all options are on the table.”
Among other efforts, Mr. Zients said that the government had now ensured that specialized syringes were shipped out with Pfizer’s vials so practitioners could extract a sixth dose from them. Dr. Albert Bourla, Pfizer’s chief executive, told investors Tuesday that the company was now two months ahead of schedule and expected to deliver a total of 200 million doses for Americans by the end of May instead of the end of July. The acceleration is at least partly because the government has decided to count Pfizer vial as six doses instead of five.
Prashant Yadav, who studies health care supply chains with the Center for Global Development in Washington, said Moderna might be able to “relatively quickly” make more of its vaccine if it received the green light to add doses to each vial.
But he said it would not be an instant change. “I don’t think Moderna has a surplus sitting around,” he said.
Mr. Yadav said the finish-and-fill process is intensely automated, devoted to warding off contamination and precise to the microgram. At top speed, as many as 1,000 vials of vaccine can be filled per minute, he said.
He said a 15-dose vial carries a trade-off: It could lead to more wasted doses if the health care professional runs out of people to get inoculated and has to throw out the rest of the doses. But in the midst of a raging pandemic, experts said, that may well be a risk that federal health officials would be willing to take.

A fast-spreading coronavirus variant first observed in Britain has gained a worrisome mutation that could make it harder to control with vaccines, Public Health England reported on Monday. And on Tuesday, a team of researchers reported an experiment suggesting that this mutation might make vaccines somewhat less effective against the variant.
The variant, known as B.1.1.7, first came to light in December. Researchers determined that it had rapidly become more common across Britain in just a couple of months.
Its spread appears to occur because of its improved ability to infect people. Experiments in test tubes suggest that some of its mutations allow the B.1.1.7 variant to hold on to cells more tightly than other coronaviruses.
Since B.1.1.7’s discovery in Britain, the variant has been reported in 72 other countries. The United States confirmed its first case of the B.1.1.7 variant on Dec. 29, but is conducting little of the genomic sequencing necessary to track the spread of new variants that have caused concern. Since then, the Centers for Disease Control and Prevention has recorded 467 samples of the variant in 32 states. Officials in New York City said on Tuesday that they had identified 13 cases of the variant and were ramping up testing capacity to detect more.
In its latest analysis, Public Health England estimated that the variant’s rate of infection is 25 percent to 40 percent higher than that of other forms of the coronavirus. Some preliminary evidence suggests that it may also cause more deaths.
Several lines of evidence suggest that vaccines will work against the B.1.1.7 variant. On Thursday, the vaccine maker Novavax announced that its British trial found no evidence that B.1.1.7 could evade the vaccine’s defenses.
But in South Africa, where a variant called B.1.351 has surged to dominance, the Novavax and Johnson & Johnson vaccines have both been less effective in trials.
That variant has been reported in 31 countries so far. In the United States, it has turned up in Maryland and in South Carolina.
Scientists suspect that the B.1.351 variant’s partial escape from vaccines is largely thanks to a single mutation, called E484K. Experiments indicate that the E484K mutation makes it harder for antibodies to grab onto the virus and prevent it from entering cells.
Now it turns out that some B.1.1.7 coronaviruses in Britain also have the E484K mutation.
To search for new mutations, British researchers reviewed the 214,159 genomes of coronaviruses that the United Kingdom has sequenced as of Jan. 26. In its report, Public Health England said that they found 11 samples of the B.1.1.7 variant that also had the E484K mutation.
Since that analysis, more of these viruses have come to light. NextStrain, a website where scientists gather and analyze coronavirus genomes, now identifies 16 B.1.1.7 variants that carry the E484K mutation.
These B.1.1.7 coronaviruses gained the mutation thanks to random copying errors as they multiplied inside of people. The evolutionary tree of the coronaviruses suggests that 15 of the variants descend from one common ancestor that gained the E484K mutation. Meanwhile the sixteenth variant seems to have gained the same mutation on its own.
Commenting on Monday’s report, Kristian Andersen, a virologist at Scripps Research Institute in La Jolla, Calif., said that it was impossible yet to say whether the E484K mutation would make these coronaviruses not only more contagious but more resistant to vaccines. “It’s much too early to speculate whether it will, so we’ll have to wait for data,” he said.
Just because the E484K mutation helps the B.1351 variant, the one initially found in South Africa, evade antibodies doesn’t mean it will do the same in other variants. That’s because mutations don’t have a fixed effect. The impact of a single new mutation to a virus depends on the other mutations that the variant already carries.
But in a report posted online Tuesday, Rajiv Gupta, a virologist at the University of Cambridge, and his colleagues reported an experiment they ran to address exactly this question. They combined the E484K mutation with other key mutations found in the B.1.1.7 variant, the one initially found in Britain. The addition of the E484K mutation made it difficult for antibodies to block the viruses. The researchers wrote that they “observed a significant loss of neutralizing activity.”
However, Dr. Gupta and his colleagues used antibodies taken from people who had received just the first of two doses of the Pfizer-BioNTech vaccine. It remains to be seen whether the B.1.1.7 variant with the new mutation, E484K, can evade antibodies after a full vaccination.
Nicholas Davies, a mathematical biologist at the London School of Hygiene and Tropical Medicine, cautioned that with so few of these new coronaviruses, it’s hard to say whether they will become more common than ordinary B.1.1.7 variant.
But it is striking that the same mutation, E484K, has now been documented arising several times in Britain, as well as in South Africa. Meanwhile, in Brazil, yet another variant has also gained the same E484K mutation on its own.
Dr. Davies speculated that the mutation may give the virus an advantage when it is spreading in populations where a lot of people have already been sick with Covid-19. It may be able to evade their antibodies to other variants. “E484K may well convey a fitness advantage in settings where there is existing immunity,” Dr. Davies said.
If so, the virus may be providing the world with a dangerous new example of a common theme in evolution. A good solution can arise more than once — such as flight, which evolved in birds, bats, and insects. Evolutionary biologists call this repeated pattern convergence.
“It’s not great to see this mutation in the B.1.1.7 lineage, although I think it’s no surprise at all,” said Dr. Andersen. “We should expect that to happen.”
Dr. Gupta argued on Twitter that the best defense against this convergence is vaccination. By making it harder for coronaviruses to get from person to person, they will have fewer chances to gain the E484K mutation or other dangerous changes.
“We need to continue vaccinating and drive down transmission,” Dr. Gupta wrote.

Portugal, struggling to contain an outbreak that has led to the highest death rate in Europe, has rapidly filled the beds in intensive care units established for Covid-19 patients and is being forced to create spaces by diverting spaces meant for other critical care patients.
With emergency rooms overwhelmed, particularly in the capital region of Lisbon, hospitals have asked patients to try to treat themselves at home, and the government has reached out to other European countries for assistance.
Some patients have also been airlifted to island hospitals or moved to hospitals in regions not as badly affected.
For those who do show up at hospitals in Lisbon, they are finding a system on the brink, with scores of people lining up outside and waiting to be diagnosed in idling ambulances parked outside.
“We are managing the full capacity of the country,” Pedro Siza Vieira, the Portuguese economy minister, said in a phone interview on Tuesday. While new infections appeared to be falling in some regions, the outbreak in Lisbon was still raging.
“We are looking at a couple of weeks that will be difficult,” he added.
Mr. Siza Vieira himself contracted Covid-19 last month, and about a third of the government’s ministers have also caught the illness recently, or had to isolate after being in contact with a person who had Covid-19.
Portugal, a nation of about 10 million on the Iberian Peninsula, is in the grips of its worst crisis of the pandemic, and 5,000 people died in January.
During the first wave of Covid-19, Portugal was one of the success stories of Europe, after applying a strict lockdown that helped keep its death toll low, particularly in comparison with neighboring Spain.
But since Christmas, Portugal has faced a surge in infections and fatalities.
Government officials have said that the crisis has been amplified by the spread of the Covid-19 variant first discovered in Britain. President Marcelo Rebelo de Sousa of Portugal told a recent news conference that the variant accounted for more than 50 percent of new infections in his country.
Mr. Siza Vieira, the economy minister, said on Tuesday, “We don’t have evidence of the Brazilian variant being significantly active in Portugal while we have evidence that the U.K. variant explains more than half of new cases, particularly in the Lisbon area.”
However, British officials have expressed their own concern about the spread in Portugal of the variant first discovered in Brazil, leading Britain to announce travel restrictions on its European neighbor.
The travel ban was part of wave of new border closures around the world as countries raced to vaccinate their populations while trying to limit the spread of new variants.
Whatever is driving infections in Portugal, new cases are only now starting to show signs of slowing after a national lockdown was reinstated in mid-January.
The defense minister in Germany, Annegret Kramp-Karrenbauer, said that the country was preparing to dispatch army personnel and equipment to Portugal, noting that all of those deployed would be vaccinated.
And Chancellor Sebastian Kurz of Austria announced on Twitter that his country would welcome some patients transferred from Portugal, without detailing how many.
Ricardo Baptista Leite, an opposition lawmaker in Portugal who is also a medical doctor and head of the public health department at the Catholic University of Portugal, said he was grateful for the support.
“We now have international aid coming in to try to save as many lives as we can,” he said. “But the time will come to assess what went wrong.”
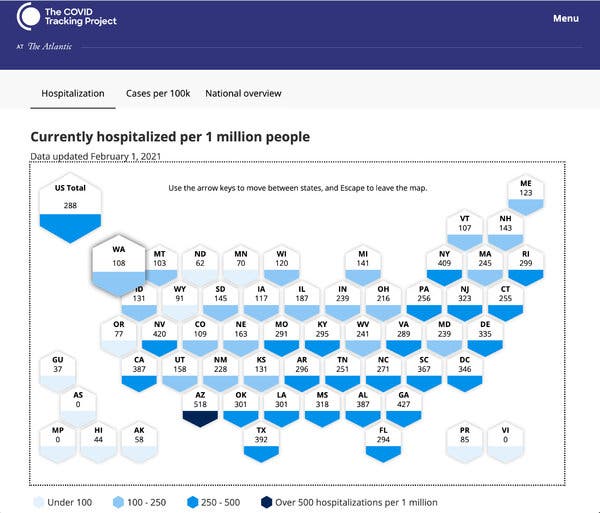
The Covid Tracking Project, one of the most trusted sources of coronavirus-related public health data in the U.S., announced this week that it will shut down soon, with its last daily updates coming on March 7, the anniversary of its founding.
Hosted by The Atlantic magazine and run by a small team of employees and scores of volunteers, the project is one of a number of nongovernmental efforts that sprang up over the past year to track and document with hard data the scope and trajectory of the coronavirus epidemic, one of the worst public health crises in the nation’s history, as well as the contours of the government response.
It was particularly noteworthy for keeping close tabs on coronavirus hospitalizations, as well as data on testing and newly reported cases in individual states and the nation.
The project’s co-leaders, Erin Kissane and Alexis Madrigal, said in a statement posted to its website that the federal government was now providing more of the essential real-time pandemic data than the project has been offering. They also noted that a number of nongovernment sources were still delivering “outside checks on federal public health data.” Among the sources they mentioned were a federal vaccine tracker website and a county-level hospitalization tracker run by the University of Minnesota.
“Although substantial gaps and complexities remain, we have seen persuasive evidence that the C.D.C. and H.H.S. are now both able and willing to take on the country’s massive deficits in public health data infrastructure, and to offer the best available data and science communication in the interim,” they wrote, referring to the Centers for Disease Control and Prevention and the U.S. Department of Health and Human Services.
The Coronavirus Tracking Project’s data has been cited extensively by researchers, by the news media (including The New York Times), and by the government itself. The project grew out of an article that Mr. Madrigal, a staff writer for The Atlantic, reported in March along with a colleague, Robinson Meyer.
At that time, Trump administration officials were boasting about setting up a robust testing program. The journalists called every state, and could only verify that 1,895 people had been tested up to that point.
Bloomberg Businessweek wrote in November that the project was “in some ways the quintessential pandemic-era startup, existing almost entirely on Slack, the workplace messaging platform.” The headline called the participants, many of whom have never met in person, “data heroes.”
Mr. Madrigal, in an interview on Tuesday, seemed to agree.
“It’s been a real source of strength to see all these different people pull together, even at a time when the country was flailing,” he said.
After the project’s final daily update on March 7, Mr. Madrigal and Ms. Kissane said, the project would spend two more months on “documentation, analysis and archival work” before shutting down entirely in May.
Iran announced on Tuesday that its first batch of Covid-19 vaccine, Russia’s Sputnik V, would arrive on Thursday. It coincided with a study published Tuesday in a British medical journal that the vaccine was safe and effective.
The news could not come at a better time for Iran. Vaccines, like everything else in the Islamic Republic, have been politicized and subject to disinformation from the top down.
Iran’s supreme leader has declared a ban on importing American- and British-made vaccines, saying they could not be trusted. The Iranian health ministry canceled a batch of donated Pfizer doses and said it would import vaccines from Russia and China and co-develop a vaccine with Cuba.
For weeks, senior Iranian health officials have publicly argued over whether it was safe to inoculate Iranians with the Russian vaccine because it had not been approved by the World Health Organization or any Western medical agency.
Before the Lancet study said that Sputnik V had a 91.6 percent efficacy rate against the coronavirus, several high-profile Iranians had expressed skepticism about it, including Iran’s top infectious disease official and the head of Parliament’s health committee. And 98 physicians from Iran’s largest union of medical workers wrote a letter last week to President Hassan Rouhani urging him not to purchase the “unapproved and unsafe” Sputnik vaccine.
Other health officials pushed back defending the Russian vaccine, including the health minister and his former spokesman.
On Twitter, a hashtag trending in Persian was #buysafevaccines, as many Iranians declared they would reject Russian- and Chinese-made vaccines.

A military judge on Tuesday indefinitely postponed the arraignment of three prisoners at Guantánamo Bay who were scheduled to make their first court appearance in 17 years of detention, finding that the coronavirus pandemic made it too risky to travel to the Navy base.
The Indonesian prisoner known as Hambali, who has been held since 2003 as a former leader of a Southeast Asian extremist group, and two accused accomplices were scheduled to appear at the war court on Feb. 22. But Col. Charles L. Pritchard Jr., the military judge who was to travel to Guantánamo this week, ruled that “the various counsels’ belief that their health is at significant risk by traveling” to the base in Cuba was reasonable.
Colonel Pritchard is the most recent military judge to join the Guantánamo military commissions bench, and the latest to postpone a proceeding as too risky in nearly a year of cancellations over the coronavirus. Pretrial hearings in the death-penalty case against five men accused of plotting the Sept. 11, 2001, attacks have been delayed for a year.
The judge, court staff and lawyers bound for the hearing began a quarantine in the Washington area over the weekend before a charter flight to the base on Thursday.
Once there, the passengers were to be individually quarantined for 14 days under a plan devised by prosecutors to protect those living on the base of 6,000 residents and at the prison from the threat of infection.
The case had been dormant throughout the Trump administration, but on the second day of the Biden administration, a senior Pentagon official appointed under the Trump administration who had been put in charge of the military commissions approved the prosecutions.
The defendants include Mr. Hambali, who is charged as Encep Nurjaman and is the former leader of the extremist group Jemaah Islamiyah, and his accused accomplices, Mohammed Nazir Bin Lep and Mohammed Farik Bin Amin, who are Malaysian.
The three men were captured in Thailand in 2003 and are charged with conspiring in the 2002 nightclub bombings in Bali, which killed 202 people, and the 2003 Marriott Hotel bombing in Jakarta, which killed at least 11 people and wounded at least 80. They spent their first three years in the C.I.A.’s secret prisoner network before they were transferred to Guantánamo for trial in 2006.
GLOBAL ROUNDUP

NEW DELHI — Mayandi Soundara Raj, an engineer pictured astride a motorcycle, was “a perfect husband” to his wife. He died on July 10.
Arkadipta Basu, who died on Sept. 17, showered her family with “love and affection,” according to Anindya Basu, her husband.
“I couldn’t keep my promise to be with you forever,” he writes under a photograph of her in a bright red sari.
Mr. Raj and Ms. Basu are among those memorialized on a new website dedicated to people in India who have died of virus-related illnesses, who number more than 154,000.
At the height of India’s outbreak last fall, more than 1,000 people were dying every day. As in many other places, pandemic restrictions often meant that friends and family members were unable to attend funerals or be present for last rites.
“As a society, we probably couldn’t provide them the dignity in which we would have loved to bid them a farewell,” said Abhijit Chowdhury of the Covid Care Network, a nonprofit group in the eastern city of Kolkata that established the site.
The group says it hopes that the memorials will broadly represent India’s population, and invites submissions online that are verified with death certificates. “We are initiating this, but we hope this becomes a place where everybody in the country could join,” Mr. Chowdhury said.
India appears to be experiencing something of a breather in its outbreak. The country has registered more than 10 million total cases, the second-highest tally in the world, after that of the United States, according to a New York Times database. Compared with almost 100,000 cases a day last fall, India now has a seven-day average of about 12,000 new daily infections. The country of 1.3 billion people has also begun one of the world’s largest inoculation campaigns, with about 3.9 million health care workers having received their first dose of the coronavirus vaccine by Tuesday afternoon.
In other developments from around the world:
-
New Zealand’s drug regulator said on Wednesday that it had provisionally approved the Pfizer-BioNTech vaccine but added 58 conditions, most of which require the manufacturer to supply extra data. Pfizer said last week that the first of the 1.5 million vaccines on order were expected to arrive before the end of February. New Zealand’s director-general of health, Ashley Bloomfield, has said that the country will be “ready to start vaccinating people as soon as a vaccine arrives.”
-
The state of Victoria, Australia, declared the coronavirus “technically eliminated” on Wednesday — for the second time — after a month in which no new cases were recorded. The state government also announced a rollout plan for the Pfizer-BioNTech vaccine that is scheduled to begin in late February. Frontline health workers will be among those included in the first phase, and nine vaccination hubs will be established at the state’s major hospitals. “This is a significant day in the response of the Victorian community to the pandemic,” said Martin Foley, the state’s health minister. “It turns a corner.”
-
Local officials in Taiwan rescinded a $3,500 penalty issued to a man for violating quarantine after it was found that he had been forced to leave his quarantine premises against his will. He had been kidnapped by debt collectors who thought he was someone else.
-
Prime Minister Yoshihide Suga of Japan said on Tuesday that the state of emergency in Tokyo and nine other prefectures would be extended by one month, to March 7. Mr. Suga said that while new infections had declined from their peak last month, the health care system was still strained.

Andrew Yang, who ran for president last year and is now a leading New York City mayoral candidate, announced on Tuesday that he had tested positive for the coronavirus.
“After testing negative as recently as this weekend, today I took a Covid rapid test and received a positive result,” Mr. Yang said in a statement. “I am experiencing mild symptoms, but am otherwise feeling well and in good spirits. I will quarantine in accordance with public health guidelines and follow the advice of my doctor.”
More than perhaps any rival in the race, Mr. Yang has been campaigning extensively in person despite the pandemic, holding numerous events outdoors since announcing his candidacy last month.
His approach has generated voter enthusiasm and attracted media attention, but with clear risks. A member of his staff tested positive less than a week after he announced his candidacy.
Two other mayoral candidates, Scott M. Stringer, the city comptroller whose mother died of complications from Covid-19, and Maya Wiley, a former counsel to Mayor Bill de Blasio, have had to quarantine recently, though they have generally been more cautious about in-person campaigning.
An entrepreneur with no previous experience in electoral politics, Mr. Yang mounted a long-shot bid for the Democratic presidential nomination in 2020 that raised his public profile and won him many fans, especially among younger voters. But he never became a significant factor in the race, and dropped out after the New Hampshire primary.
In his statement on Tuesday, Mr. Yang said, “I will continue to attend as many virtual events as possible,” and said he looked forward to hitting the trail again “when the time is right.” He said his campaign has begun the contract-tracing process.
The drug maker Pfizer expects its Covid-19 vaccine to generate $15 billion in revenue this year, executives said Tuesday on a call with investors to discuss the company’s fourth-quarter earnings in 2020.
Pfizer and other companies have struggled to keep up with the demand for their vaccines, as the United States and countries around the world are scrambling to vaccinate as many people as possible to combat the spread of more contagious variants.
In comments to investors on Tuesday, the company’s chief executive, Dr. Albert Bourla, said Pfizer had delivered 29 million doses to the United States by the end of January, and 65 million doses worldwide, and that it expected to manufacture up to 2 billion doses by the end of the year. It shares profits with BioNTech, the German company that developed the vaccine.
Dr. Bourla also said Pfizer now expects to deliver the 200 million doses, for about $4 billion, that it had promised to the United States two months ahead of time, by May instead of July. That accelerated timeline can be attributed in large part to Pfizer now counting six doses in every vial of vaccine that it ships instead of five — after health care providers learned they could extract an additional dose using special syringes.
“We foresee no issues with delivering the commitments we have made,” Dr. Bourla said.
And, in an indication that the coronavirus may be here to stay, Dr. Bourla said he expects the vaccine to become a standard part of its business, given the likely need for booster shots to address waning immunity to the virus as well as variants that could reduce its effectiveness.
Pfizer has said that preliminary tests of its vaccine against more contagious variants have shown that it continues to be effective, but suggested that a third shot of the same vaccine may be needed, and the company has also not ruled out changing its vaccine to address the new variants. Moderna, which has developed a similar vaccine, has said it is already working on an adapted booster shot.
“We believe it is increasingly likely that a durable Covid-19 vaccine revenue stream, like is happening in flu, is a potential outcome,” Dr. Bourla said.

Tom Moore, the 100-year-old British Army veteran whose charity walks raised $45 million and made him a national symbol of pluck during the coronavirus pandemic, died on Tuesday.
His death was announced on his Twitter account.
Mr. Moore, nicknamed Captain Tom by the British press, had been treated for pneumonia in recent weeks and tested positive for the coronavirus last month, his daughter, Hannah Ingram-Moore, said on Twitter on Sunday. He was taken to a hospital because he needed help breathing, she said, and his condition then deteriorated.
Dapper, spry and droll, Mr. Moore ambled his way into the hearts of people across Britain 82 steps at a time — the number it took to cover the length of a brick patio beside his garden in Marston Moretaine, a village an hour north of London. He did 100 laps before turning 100 last April. With backing from donors including Prince William, who called him a “one-man fund-raising machine,” Mr. Moore quickly raised 32.8 million pounds, or $45 million, for the National Health Service.
In the process, Mr. Moore became a pop-culture phenomenon, with television appearances, a book deal, a chart-topping song and a knighthood from Queen Elizabeth II, 94, who came out of seclusion to bestow the honor in July.

Democrats on Tuesday took the first step to push through President Biden’s $1.9 trillion economic rescue plan, using a budgetary maneuver that could eventually allow the measure to become law without Republican support.
The move advanced the two-track strategy that Mr. Biden and Democratic leaders are employing to speed the aid package through Congress: show Republicans that they have the votes to pass an ambitious spending bill with only Democratic backing, but offer to negotiate some details in hopes of gaining Republican support.
“We are not going to dilute, dither or delay,” Senator Chuck Schumer, Democrat of New York and the majority leader, said on the Senate floor. “There’s nothing about the process itself that prevents bipartisanship.”
The party-line vote of 50 to 49 set the stage for Democrats to advance Mr. Biden’s plan through budget reconciliation, which would allow it to pass with a simple majority vote, bypassing the need for Republican support. (Senator Patrick J. Toomey, Republican of Pennsylvania, was absent and did not vote.)
“They’ve chosen a totally partisan path,” Senator Mitch McConnell, Republican of Kentucky and the minority leader, said of Senate Democrats.
The vote came the day after 10 Republican senators met at the White House with Mr. Biden seeking a smaller, $618 billion package they said could win bipartisan backing.
Some Republican senators considered Mr. Biden receptive to their proposals, but said his chief of staff, Ron Klain, shook his head dismissively during the Republicans’ presentation, according to a participant in the meeting.
Senate Democrats could approve the budget resolution as soon as Friday. On Tuesday, a key Democratic senator announced he would support it: Joe Manchin III of West Virginia, who is a crucial swing vote, said he would agree to move forward with the budget process “because we must address the urgency of the Covid-19 crisis.”
“I will only support proposals that will get us through and end the pain of this pandemic,” Mr. Manchin said in a statement.
Mr. Manchin also reiterated his opposition to Mr. Biden’s proposal to raise the federal minimum wage to $15 an hour, which could force Democrats to drop it from their legislative package.
The budget resolution would instruct congressional committees to draft legislation that could include Mr. Biden’s stimulus proposal, which includes $1,400 direct payments for many Americans, funding for vaccine distribution, reopening schools and other measures.
More than 100 Democratic lawmakers are also urging Speaker Nancy Pelosi of California and Mr. Schumer to repeal a business tax break as part of the economic aid package. The tax cuts in question — which center on so-called net operating losses — were included in a rescue bill Congress passed in March 2020, as the pandemic spread and the nation was in the midst of a recession.
On Tuesday, an influential business group that had welcomed Mr. Biden’s initial proposal urged him to work with Republicans on a compromise — and to scale back his plans, including providing less aid for the unemployed and scrapping a call for an increase in the federal minimum wage to $15 an hour.

California state prison health authorities created a “public health disaster” by pressing lower-ranking prison workers to hastily transfer inmates between prisons in the middle of a coronavirus outbreak, according to a watchdog report released this week.
The transfers in May 2020, which were supposed to shift medically vulnerable prisoners away from danger, wound up igniting deadly new outbreaks at the facilities where they were sent, including San Quentin State Prison.
The report by the California Office of the Inspector General found that authorities in the California Correctional Health Care Services, which oversees most of the state’s prison health care, ignored or overruled multiple warnings about the dangers of transferring the inmates.
“Our review found that the efforts by C.C.H.C.S. and the department to prepare for and execute the transfers were deeply flawed, and risked the health and lives of thousands of incarcerated persons and staff,” the report concluded.
San Quentin had not reported any cases of coronavirus before late May, when officials ordered the transfer of 122 inmates to San Quentin from another state prison, the California Institution for Men in Chino. Since then, more than 2,240 San Quentin inmates have been sickened with the virus and 28 died, according to state data.
Sixty-seven inmates from the Chino prison were transferred to Corcoran State Prison, where a smaller outbreak later occurred, the report said.
The inspector general found that 91 of the 122 inmates who were sent to San Quentin eventually tested positive, and two died.
In an email response to a request for comment, the state prison health care system acknowledged making “some mistakes.”
The transfers, the statement said, “were based on a thoughtful risk analysis using scientific information available in May 2020 concerning transmission of this novel disease. We have acknowledged some mistakes were made in the process of these transfers.”
The statement said the prison system had made pandemic safety improvements, including “increased testing, the use of designated isolation and quarantine spaces, and the enhanced use of personal protective equipment when indicated.”
The inspector general’s report, released on Monday, found that prison health authorities overruled dire warnings from a number of nurses and other lower-ranking health officials about the transfers, including after the authorities were informed that only three of the 189 inmates scheduled for transfer had been recently tested.
When concerns were raised about packing dozens of inmates into prison buses for a six-hour trip, a state prison health official brushed them aside, writing, “the situation appears to outweigh the risks.” The report did not name the official.
Marion Wickerd, whose husband, a prisoner at San Quentin, was infected in June, said state prison authorities needed to be held accountable.
“The transfers were inhumane, and someone needs to take responsibility for what happened,” she said. “It was wrong. It was dead wrong.”

As new virus cases and hospitalizations drop and increasing attention turns to the state’s vaccine rollout, California officials are attempting a fine balancing act between speeding the process up and ensuring that vulnerable populations aren’t shut out.
On Tuesday, Dr. Mark Ghaly, the state’s secretary of health and human services, said in a news conference that the two goals are not mutually exclusive.
“This notion that we have to make a choice between speed and equity — it’s a false choice,” he said. “We can do both.”
But as has been the case throughout the fraught vaccine distribution in the nation’s most populous state, the details of how both of those goals can be achieved are more difficult to pin down.
So far, more than 3.5 million doses of vaccine have been administered in California, Dr. Ghaly said. The rate of vaccinations statewide, he said, has been building day by day, since a holiday surge of hospitalizations. According to a New York Times tracker, that’s about 7.2 percent of the state’s population — somewhere in the middle of the pack of states.
However, the state has not yet released demographic statistics about who received the vaccines, so it’s unclear whether Latinos or other Californians of color who have been at disproportionate risk have been vaccinated at commensurate rates.
Dr. Ghaly suggested state officials were weighing several methods of incentivizing vaccine providers to specifically target vulnerable communities, including paying them to get vaccines into harder hit neighborhoods, rather than forcing residents to go to mass vaccination sites that may be farther away.
Gov. Gavin Newsom last week announced that the state would revamp its vaccine distribution approach in the wake of widespread criticism that the rollout has been confusing and piecemeal.
Part of that restructuring involves a plan to enlist two of the state’s biggest health insurers, Blue Shield of California and Kaiser Permanente, to help with a statewide distribution system that would prioritize equity and would help streamline a patchwork system.
And the state launched a website and data portal that officials have said will not only notify Californians when they are eligible for a vaccine and help them make appointments to do so, but would also help collect and share data with federal officials or others who may be working to allocate vaccines.
Experts have said that enlisting bigger, more experienced health care providers could help accelerate a vaccine rollout that has been hampered by the fact that it’s been implemented by already overwhelmed local public health departments.
On Tuesday, though, Dr. Ghaly repeatedly declined to share details of the partnerships, beyond that any transitions to new systems would not disrupt existing appointments.