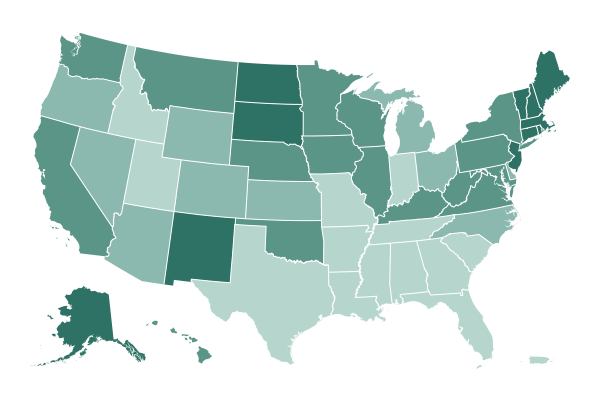
Texas, Indiana and Georgia announced Tuesday that residents 16 years and older will be eligible for Covid-19 vaccinations starting Thursday for Georgia residents, Monday for Texans and on March 31 for Indianans. They joining a growing list of states that plan to broaden vaccine eligibility to all adults ahead of a May 1 deadline set by President Biden.
“With every dose, Texas gets closer to normal and protects more lives from COVID-19 hospitalization and death,” the state’s health department said in a Twitter post.
West Virginia, Alaska and Mississippi are the only states where all adults are eligible to receive shots. Others, like Texas, Georgia and Indiana, have announced future expansions; Utah, for example, will open eligibility to all adults on Wednesday. And Tennessee announced last week that all residents 16 and older would be eligible for vaccinations starting April 5. Some states, such as New York, have been gradually expanding eligibility: New Yorkers 50 years and older became eligible on Tuesday.
Widening the eligibility for vaccines comes at a time when federal health officials have warned of a possible fourth surge of the virus as troubling new variants spread, urging Americans to get vaccinated. Mr. Biden has said there would be enough vaccines available by the end of May for all adults and has suggested that Americans could see a return to normalcy by July 4 if they got vaccinated and kept following health precautions, like mask wearing.
Virus case numbers in the country “have plateaued — that’s not good, they should keep going down and down,” Dr. Anthony S. Fauci, the country’s leading expert on infectious diseases and a top adviser to Mr. Biden, said Tuesday on “Good Morning America.” As of Monday, the seven-day average of new virus cases nationwide was 54,000 a day, according to a New York Times database, a level comparable to that of mid-October.
“When you plateau like that, there really is a danger of a resurgence,” Dr. Fauci said.
About 25 percent of the total U.S. population has received at least one shot, and 14 percent are fully vaccinated. The pace of vaccinations has been steadily increasing in recent weeks to an average of about 2.5 million shots daily, well above the daily rate of fewer than one million shots two months ago. The White House press secretary, Jen Psaki, said Tuesday that a total of 27 million doses would be allocated to states, pharmacies and other jurisdictions and programs, 4 million of which would be of the Johnson and Johnson vaccine.
In Texas, about 22 percent of all residents have received at least one shot of a vaccine, and 11 percent are fully vaccinated, according to a New York Times analysis of data from the Centers for Disease Control and Prevention.
Earlier this month, Gov. Greg Abbott lifted the state’s mask mandate and scrapped crowd capacity limits on all businesses, a move that drew criticism from federal officials, including the president. “Texas is OPEN 100%,” Mr. Abbott tweeted at the time.
About 19 percent of Georgians have received at least one dose of a vaccine, according to a Times database. Gov. Brian Kemp said that as of Tuesday, more than one million older residents have received at least one vaccine dose.
“As you all know, this is our ticket back to normal,” Mr. Kemp said. “And we’re getting closer to that point every single day.”
Businesses in much of the country are open and more than a dozen states have no mask mandates in, a Times database showed.
In Indiana, Gov. Eric Holcomb announced that the state’s mask mandate will drop to an advisory on April 6. But face coverings will remain mandatory in all state buildings, facilities, vaccination and testing sites “until further notice” and in schools for the remainder of the year.
In North Carolina, Gov. Roy Cooper said Tuesday that the state would ease some restrictions beginning on Friday, including increasing the number of people permitted at mass gatherings, and reinstating normal hours for when alcohol may be served at bars and restaurants. Mr. Cooper continues to enforce a statewide mask order.
In Virginia, Gov. Ralph Northam announced some changes on Tuesday, including allowing sports and entertainment venues to operate with additional capacity and increasing the number of people allowed at events indoors and outdoors. He also issued preliminary guidance on in-person graduation ceremonies and commencements and will continue universal mask wearing.
On Monday, the C.D.C. director, Dr. Rochelle Walensky, said members of Mr. Biden’s virus team have reached out to governors and other state officials about the spread and detection of variants and sought to persuade them “to slow down the relaxation.”
“We just don’t want to be at this rapid uptick of cases again, and that is very possible that that could happen,” she said. “We’ve seen that. We’re behind the eight ball when that starts to happen.”
Only hours after AstraZeneca announced encouraging news about the effectiveness of its Covid-19 vaccine on Monday, a group of medical experts charged with monitoring the company’s clinical trial made a highly unusual accusation: AstraZeneca had essentially cherry-picked data to make its vaccine look better.
The accusation, in a two-page letter sent Monday to the company and federal officials, was a fresh blow to the credibility of a vaccine whose low price and relatively easy storage have made it critical to the global fight against the pandemic.
The private letter, which was described by people who have read it, castigated AstraZeneca for jeopardizing the integrity of a closely watched clinical trial.
“Decisions like this are what erode public trust in the scientific process,” the board wrote.
The letter prompted the National Institute of Allergy and Infectious Diseases to issue a sharply worded statement shortly after midnight on Tuesday, making public the panel’s concerns.
The fight is about the degree of effectiveness of a vaccine that is undisputably effective.
While AstraZeneca said on Monday that its vaccine appeared to be 79 percent effective at preventing Covid-19, the panel of independent experts said the actual number may have been between 69 percent and 74 percent. The mass availability of a vaccine with even a 69 percent efficacy rate could help the world conquer the coronavirus.
But the public airing of a conflict between a pharmaceutical company and a board overseeing a clinical trial is almost unheard-of. It is certain to trigger extra scrutiny of the vaccine by the Food and Drug Administration and other regulators if, as expected, AstraZeneca seeks their authorization to use it on an emergency basis in the United States.
Repeated problems with the data presented by AstraZeneca have shattered the confidence of American regulators and threaten to cast a worldwide cloud over the company’s vaccine.
“Any type of thing like this could unfortunately contribute to a lack of confidence in the process,” said Dr. Anthony S. Fauci, the nation’s leading infectious disease expert.
Fears that the vaccine might trigger rare but serious side effects had led more than a dozen countries, mostly in Europe, to temporarily suspend the use of the shot. European regulators last week affirmed the vaccine’s safety. The results from the U.S. trial on Monday seemed to validate the vaccine’s safety and made it look more effective than in earlier trials.
In short, it bolstered the credibility of arguably the world’s most important vaccine, one that has been authorized for use in more than 70 countries. But the overnight announcement from the institute immediately raised a new set of questions about it and AstraZeneca.
But the overnight announcement from the institute immediately raised a new set of questions about it and AstraZeneca.
At issue is whether to count, in the public release of data, more recently confirmed cases of Covid-19 among participants. The oversight board pushed AstraZeneca representatives last week to go through a backlog of potential cases and classify whether they were or were not Covid-19. That had the potential to affect the vaccine’s effectiveness rate, for better or worse.
The requested analysis showed that the vaccine was between 69 percent and 74 percent effective, according to the oversight board’s letter.
But when AstraZeneca unveiled its interim results on Monday, the company did not count those newly classified Covid-19 cases. As a result, it reported that the vaccine appeared to be 79 percent effective at preventing the disease.
Until they received the monitoring board’s letter, AstraZeneca executives weren’t aware that the panel expected them to include the updated figures in their news release, according to a person familiar with the executives’ thinking.
Federal officials, however, were stunned to discover on Monday that AstraZeneca had released those results even though the monitoring board had spent days pushing for — and eventually received — updated data. By then, Dr. Fauci had publicly hailed the results at a White House briefing, and the company had been showered with positive media coverage. AstraZeneca’s shares rose about 4 percent on Monday.
AstraZeneca on Tuesday defended its actions, saying the interim results appeared to be “consistent” with more recent data collected during the trial. The company said it would immediately share its latest data with the monitoring board and reissue fuller results within 48 hours. The company’s shares fell 3.5 percent on Tuesday.
AstraZeneca’s relationship with the U.S. authorities has been fraught since last year, when senior health officials believed the company was not being forthright about the design of its clinical trials, its results and safety issues. That skepticism carried over to last week, when senior officials at a number of federal health agencies grew suspicious about why AstraZeneca had not announced data from its U.S. study.
Last week, an apparent delay in the release of the vaccine’s interim trial results raised fresh suspicions among federal officials.
In response to questions from The New York Times, an AstraZeneca spokeswoman, whom the company refused to name, said on Friday that any back and forth between the company and the monitoring board was simply run-of-the-mill dialogue.
“As is often the case,” the spokeswoman said, monitoring boards “can request new or clarifying analyses of data from the trial. This would enable them to ensure the robustness of their determinations.”
The U.S. trial, which involved more than 32,000 participants, was the largest test of its kind for the shot. The results AstraZeneca released on Monday were from an interim look at the data after 141 Covid-19 cases had turned up among volunteers.
The announcement this week that the AstraZeneca shot, the workhorse of global vaccine rollouts, had achieved nearly 80 percent efficacy in a gold-standard American trial was met with relief by the many countries relying on it.
But by Tuesday, that campaign had, once again, been thrown off course, at least for the moment. For AstraZeneca, it was seemingly another episode of public relations whiplash, part of a series of recent miscues and communication blunders by the company that scientists said had undercut the effort to sell one of the most potent and indispensable vaccines against the coronavirus.
In a highly unusual move, American health officials said on Tuesday that the company’s account of its U.S. trial findings had not been entirely accurate, suggesting that AstraZeneca had used only the most favorable data to generate apparently spectacular efficacy results.
Those comments created new friction between AstraZeneca and American officials even as the company vies for a coveted authorization from the Food and Drug Administration. But more urgently, they threw a wrench into the efforts of elected leaders around the world to rebuild trust in a shot whose low price and easy storage requirements have made it the backbone of many countries’ campaigns to end the pandemic.
“It’s eroding confidence,” said Simon Clarke, an associate professor in cellular microbiology at the University of Reading. “When you pump things up, and then people not unreasonably question it, then that erodes confidence.”
Faith in the vaccine had already plunged across Europe after recent reports that a very small number of recipients had developed unusual blood clots. In France, Germany, Italy and Spain, more people now believe that the vaccine is unsafe than that it is safe, polling has shown, a blow to a shot that remains the continent’s best hope for saving people’s lives during a mounting surge of new infections.
Despite the drumbeat of troubling news reports about the vaccine, European and global regulators have deemed it safe and effective. More than 11 million doses have been administered in Britain alone, almost all of them without serious side effects, driving down hospitalizations and helping the country to emerge from a dreadful wintertime wave of infections.
Nevertheless, results from AstraZeneca’s U.S. trial was hotly anticipated. Health officials around the world were looking to it as a crucial guide to their own rollouts: It would supply crucial data on older people, who had not been as well represented in earlier trials, and a more precise read on the vaccine’s overall efficacy, which had appeared from earlier trials to be lower than that of other leading shots.
By Tuesday, scientists said, it seemed as though AstraZeneca had punched a hole in fledgling efforts by lawmakers to shore up public confidence in the vaccine. Rather than sewing up questions about the shot, it had called to mind communications problems that have dogged the company since last year, delaying the regulatory process in some regions and creating hesitation among some recipients.
In its first public comments, AstraZeneca said that the results published on Monday reflected its U.S. trial data up to Feb. 17. It said that its preliminary assessment of more complete trial data showed that “the results were consistent with the interim analysis,” but said that it would share more up-to-date efficacy results within 48 hours.
Scientists said that the problem could yet turn out to be a technical matter that did not change their assessment of the vaccine. American officials did not suggest that any safety issues had been withheld, a subject of intense interest in the wake of the concerns in Europe.

MOSCOW — President Vladimir V. Putin of Russia was inoculated against the coronavirus on Tuesday, the Kremlin said, ending months of holding out against being vaccinated even while promoting his country’s domestically made shots.
The president’s injection was not shown on television, an exception to the usual wall-to-wall coverage of Mr. Putin’s daily activities on state television.
His spokesman, Dmitri S. Peskov, said that Mr. Putin had promoted vaccination in other ways and did not need to be shown in public receiving a shot.
“The president, as you see, has dedicated a significant part of his work time to events, discussions and meetings about vaccines, about production of vaccines and so on,” Mr. Peskov said. “The president is already doing a lot for vaccine propaganda.”
Mr. Peskov addressed the issue of shortage only in export markets for the most widely used Russian vaccine, called Sputnik V, saying that demand abroad exceeded supply and that the vaccine therefore did not need promotion.
In addition to Sputnik V, Russia has approved for emergency use two other domestically developed vaccines — EpiVacCorona and CoviVac — that have yet to complete their clinical trials. All three Russian vaccines require two doses.
Mr. Peskov declined to specify which of the three Russian-made vaccines Mr. Putin received.
Under Russian rules, Mr. Putin became eligible for a Sputnik V shot in late December based on his age; he is 68. But months passed with no word from the Kremlin on his inoculation.
The Kremlin and outside analysts of Russian vaccine politics have offered a variety of explanations about the presidential foot-dragging.
At the least, Mr. Putin is not shy about appearing in public shirtless, relishing opportunities to show his general good health with bare-chested pictures from Siberian fishing or horseback-riding vacations.
He is also not known to be generally squeamish about vaccines. He told Russian newspaper editors last month that he receives annual flu shots, and said then that he might be inoculated against the coronavirus along with a flu shot in the autumn.
In response to a question on whether he should promote vaccination by example, he said he didn’t want to “make a monkey” of himself by appearing in public receiving a coronavirus vaccine, according to a report of the meeting.
Russia’s inoculation campaign has lagged far behind the United States and most European nations — the country has vaccinated 3.9 percent of the population with at least one dose, compared with 25 percent in the United States. Some attribute that difference to widespread vaccine hesitancy, something a presidential shot could help overcome.
Russian news media reports, though, have pointed to signs of another cause: shortages and production bottlenecks that officials have only recently acknowledged.
During a video conference with vaccine makers on Monday, Mr. Putin said Russia had produced 8.9 million two-dose sets of the Sputnik V vaccine since regulators approved it in August. But he said production would ramp significantly to 17 million sets monthly starting in April. Last fall, officials had predicted a far swifter rollout.
Promoting vaccination with a presidential shot before doses were widely available in Russia might have served only to highlight the vaccine shortage at home even as Russia has been exporting vaccines globally, a sensitive political issue.
Mr. Putin cited his own vaccination plans on Monday while also announcing plans to produce enough doses for most adults by August.
global roundup

Chancellor Angela Merkel of Germany, warning that her country is facing a significantly more deadly wave of the coronavirus, announced a five-day lockdown over Easter and the extension of existing restrictions until mid-April in an effort to break a spike in coronavirus cases.
Starting April 1, until the following Monday, Germany will effectively shut down for an extended Easter break, with private meetings limited to only two groups of up to five adults and all stores ordered shuttered, with only supermarkets allowed to open on the Saturday. Churches are asked to hold services online, and people are being asked to stay home and not travel.
“We are in a very, very serious situation,” Ms. Merkel told a news conference early Tuesday, after hours of deliberations with the leaders of the country’s 16 states over the Easter lockdown and extension of existing restrictions through April 18.
“After we were able to sharply bring down the number of new infections in January, we are now experiencing, through the spread of the more contagious British variant, a more dangerous variation, the numbers are going up and the intensive care beds are filling up,” she said.
Germany is the latest country in Europe to tighten restrictions as more contagious virus variants spread and the continent struggles to vaccinate its citizens. Poland, Italy and parts of France have ordered that residents stay home, and many businesses have shut ahead of the holiday.
A resurgent virus and lagging vaccinations have forced governments to renege on promises that they would slowly reopen businesses and society as spring approached. That has spurred protests across Europe as people chafe at more restrictions.
Europe’s vaccine campaign slowed after a small number of cases of blood clots and abnormal bleeding were reported in patients who received the AstraZeneca vaccine, dampening confidence in its safety. While the European drug regulator, the European Medicines Agency, cleared the vaccine for use last week and said it was “safe and effective,” the scare further complicated vaccination efforts.
Just three weeks ago, Ms. Merkel and state officials hammered out a road map to reopening that relied on a decline in case rates. But the number of new daily cases in Germany has increased by 69 percent in the past two weeks, to levels last seen in January.
-
Residents of England who travel abroad without a valid reason will be fined 5,000 pounds, or $6,900, under coronavirus regulations that are scheduled to come into force on Monday if lawmakers approve. Daily coronavirus deaths in Britain have dropped to their lowest level since fall, thanks in part to a vaccination program that has already reached more than half the adult population, and the country is preparing to slowly reopen its economy after months of national lockdown. A stay-at-home order is to be lifted on Monday, though many shops and other businesses will be closed until mid-April or later. Travel abroad for leisure is banned until May 17 at the earliest, and the new regulations signal a potentially longer wait for vacationers.
-
A year after European leaders ordered people into their homes to curb a deadly pandemic, thousands are pouring into streets and squares. Often, they are met by batons and shields, raising questions about the tactics and role of the police in societies where personal liberties have already given way to public health concerns. From Spain and Denmark to Austria and Romania, frustrated people are lashing out at the restrictions on their daily lives.
-
Health authorities in Greece announced a record in daily coronavirus cases on Tuesday, just a day after the country’s government relaxed some lockdown restrictions. The country’s national public health organization reported 3,586 new cases on Tuesday, the highest daily rate since the beginning of the pandemic. The announcement came a day after authorities allowed archaeological sites to reopen, ahead of a planned reopening of the Greek tourism sector in mid-May.
-
Mumbai, India’s financial hub, has begun random testing for the coronavirus in malls, railway stations and other crowded places as officials attempt to tamp down on a worrying surge in cases. Rapid antigen tests will be taken without individuals’ consent, the Municipal Corporation of Greater Mumbai said in a statement on Monday. Anyone who resists will be in violation of India’s colonial-era epidemic act, which gives the government the power to fine or imprison people who violate rules to contain an outbreak.
-
The world-famous cherry trees of Japan are expected to be in full bloom in most of the country this week, but once again the coronavirus pandemic will keep the usual crowds away. Spring in Japan has long meant cherry blossoms, or sakura, with scores of picnickers welcoming the warmer weather over bento box lunches or barbecue as well as copious amounts of sake and other alcohol. But last spring, with the coronavirus spreading, officials were quick to curb cherry-blossom viewing despite its cultural significance. Yuriko Koike, the governor of Tokyo, compared the loss to “taking hugs away from Italians.”
Emily Schmall, Hisako Ueno, Mark Landler, Stephen Castle and Isabella Kwai contributed.

A monoclonal antibody treatment developed by the drug maker Regeneron sharply cut the risk of hospitalization and death when given to high-risk Covid-19 patients in a large clinical trial, the company announced on Tuesday.
The results are the latest in a growing flurry of evidence that the infused drugs, meant to mimic the antibodies that the immune system generates naturally in fighting the coronavirus, can help infected patients avoid the worst outcomes if given early.
Regeneron’s treatment, a cocktail of two antibody drugs, was given last fall to President Donald J. Trump shortly after he got sick with Covid-19 and is now one of three such therapies available in the United States.
The new results come from a Phase 3 trial that enrolled more than 4,500 patients beginning in late September, around the time virus cases began to climb dangerously in the United States. The study found that patients who got the infused treatment within 10 days of developing symptoms or testing positive had a roughly 70 percent reduced risk of being hospitalized or dying compared with patients who were infused with a placebo.
“I think these are exciting data,” said Dr. Rajesh Gandhi, an infectious diseases physician at Massachusetts General Hospital who was not involved in the study.
Even as vaccinations speed up, antibody treatments are expected to be helpful for high-risk people who still get sick for many months at least, and longer still if the virus can’t be wiped out. While there are signs that emerging virus variants may in some cases make antibodies less potent, Regeneron’s cocktail has not shown such vulnerability in laboratory tests.
In the new findings, Regeneron’s treatment worked equally well when given at half the dosing at which it was authorized. Regeneron said that it planned to request that the Food and Drug Administration allow the treatment to be given at that reduced strength.
Such a change would bring several advantages: While the cocktail is safe, getting it at a lower dose reduces the odds of side effects, such as an infusion reaction.
It would also allow Regeneron to increase the supply it can provide the United States. The company said that it had expected to supply the country with about 750,000 doses at the originally authorized higher strength by the end of June. If the lower strength is authorized, the company expects to provide about 1.25 million doses by then.
The antibody treatments from Regeneron and the drug maker Eli Lilly, which makes the other two such drugs authorized in the United States, were expected to be in high demand and to serve as a bridge in fighting the pandemic before vaccinations ramped up. Instead, they ended up sitting on refrigerator shelves in many places even during recent surges.
Many patients and their doctors did not know to ask for them or where to find them. Overwhelmed hospitals lacked the bandwidth to prioritize giving out the treatments. And some doctors were unconvinced by the relatively weak evidence available last fall supporting their use.
That picture is gradually shifting, thanks to improved logistics and more awareness. And more solid evidence, like the new data from Regeneron, also appears to be helping the drugs get used more widely. “As the data get stronger and stronger, I would expect that use will increase,” Dr. Gandhi said.
transcript
transcript
Biden Extends Obamacare Enrollment Period to Aug. 15
President Biden said on Tuesday, the 11th anniversary of the Affordable Care Act, that Americans who need health insurance would have until Aug. 15 to sign up through Healthcare.gov.
-
Eleven years ago today, President Obama signed into law the Affordable Care Act, a historic achievement that would not have been possible but for the vision and determination of one of the most successful presidents in recent American history, Barack Obama. If you enroll in Obamacare, you’re going to save an average of $50 a month, $600 a year by the reduction in payments. For millions who are out of work and have no coverage, thanks to this law, there’s an Obamacare plan that most folks can get with $0 premiums. We’re also making it easy, easier to sign up for Obamacare. We’ve opened Healthcare.gov for special enrollments on Feb. 15. In the first two weeks alone, more than 200,000 Americans gained coverage. Today, I’m pleased to announce we’ve extended that period to run through Aug. 15. With the American Rescue Plan, and the Affordable Care Act, millions of families will be able to sleep a little more soundly at night because they don’t have to worry about losing everything if they get sick.

Americans who need health insurance will now have even longer to select a health plan for the rest of the year. The Biden administration announced Tuesday that it would extend enrollment for Obamacare plans sold on Healthcare.gov until Aug. 15, from a May deadline.
The change was announced on the 11th anniversary of the signing of the Affordable Care Act, the law that established the markets where individuals could buy their own health insurance, and made plans available to shoppers regardless of pre-existing health conditions, among many other provisions.
“We have a duty not just to protect it but to make it better and keep becoming a nation where health care is a right for all and not a privilege for a few,” President Biden said at the Arthur G. James Cancer Hospital at the Ohio State University on Tuesday.
The stimulus bill signed this month expanded subsidies that help people buy such coverage, lowering the price of a typical plan to zero dollars for low-income families and offering financial assistance for the first time to households higher on the income scale. But it is taking time for the Department of Health and Human Services to implement the new provisions, and so far it has decided not to make the changes automatic. That means that even people who currently get coverage on the exchanges will need to go back to request new benefits. The extended enrollment period will give them more time to do that.
“Every American deserves access to quality, affordable health care — especially as we fight back against the Covid-19 pandemic,” said Xavier Becerra, the secretary of health and human services. His statement encouraged uninsured Americans to sign up and current Obamacare customers to review new discounts.
The administration also announced that sign-ups for special subsidies for Americans who receive unemployment insurance this year will start July 1. Congress established a system for them to receive zero-premium health plans all year, but carrying out that provision quickly has proved complicated.
Enrollment in Affordable Care Act plans for most Americans is typically limited to a six-week period each year, as a means of encouraging people to enroll when they are healthy. But the Biden administration had already opened a second enrollment period this year, arguing that the pandemic and its economic effects presented an emergency that justified expanding options for coverage. The original special enrollment period had been set to expire in mid-May.
The changes apply in the 36 states that use the federal Healthcare.gov platform to manage their insurance marketplaces. But several states that run their own marketplaces have also extended their enrollment periods.
Mr. Biden, who wore a black mask throughout his speech in Ohio, said the extension would also benefit minority communities that “historically have gone without insurance at higher rates” and have been disproportionally impacted by the coronavirus pandemic.
“Millions of families will be able to sleep a little more soundly at night,” the president said, “because they don’t have to worry about losing everything if they get sick.”
More than two months after he was fully vaccinated against Covid, a doctor in New York woke up with a headache and a dull, heavy feeling of fatigue. A fever and chills soon followed, and his senses of taste and smell began to fade.
This, he thought, could not be happening. But it was: He tested positive for the coronavirus.
“It was a huge shock,” he said. He knew that no vaccine was perfect and that the Pfizer-BioNTech shots he received had been found 95 percent effective in a large clinical trial. “But somehow in my mind, it was 100 percent,” he said.
The doctor, who requested anonymity to protect his privacy, is among the few reported cases of people who have been infected after being partly or even fully vaccinated. Nearly 83 million Americans have received at least one dose of a Covid vaccine, and it’s unclear just how many of them will have a “breakthrough” infection, though two new reports suggest the number is very small.
One study found that just four out of 8,121 fully vaccinated employees at the University of Texas Southwestern Medical Center (not Southwestern Medical Center in Dallas, as an earlier version of this briefing stated) became infected. The other found that only seven out of 14,990 workers at UC San Diego Health and the David Geffen School of Medicine at the University of California, Los Angeles tested positive two or more weeks after receiving a second dose of either the Pfizer-BioNTech or the Moderna vaccine. Both reports, published on Tuesday in the New England Journal of Medicine, show how well the vaccines work in the real world, and during a period of intense transmission.
But these breakthrough cases, though quite rare, are a sharp reminder that vaccinated people are not invincible, especially when the virus continues to circulate widely.
“We felt really strongly that this data should not lead people to say, ‘Let’s all get vaccinated and then we can all stop wearing masks,’” said Dr. Francesca J. Torriani, an infectious disease specialist at UC San Diego Health who led the California study. “These measures have to continue until a larger segment of the population is vaccinated.”
Only some of the virus-positive health workers in the California study showed symptoms, she said, and they tended to be mild, suggesting that the vaccines were protective. Some people had no symptoms at all, and were discovered only through testing in studies or as part of their medical care.
In the next few months, Pfizer and Moderna are expected to release data that should indicate how often vaccinated people become infected by the virus, even if they have no symptoms.
The vaccinated New York doctor who became ill stayed in isolation at home for nearly two weeks. He described his symptoms as relatively mild, and said that knowing he had been inoculated helped put his mind at ease.
“I didn’t have a moment’s anxiety. I did not think I was going to die. Thinking you’re not going to die — that’s a pretty big thing.”
Apoorva Mandavilli contributed reporting.

Dr. Vivek Murthy, who helped found several health-related advocacy groups and later tackled the opioid epidemic and e-cigarettes as surgeon general during the Obama administration, was confirmed by the Senate on Tuesday to reprise that role for President Biden.
The vote, 57 to 43, was a much smoother ride for Dr. Murthy than the first time he was confirmed, in 2014, when Republicans cast Dr. Murthy as a politically connected supporter of President Barack Obama’s who would use his position to push for stricter gun control. The fight dragged on for months, leaving the country without a top doctor for more than a year.
When President Donald J. Trump was elected, Dr. Murthy was asked to resign. He refused and was fired, his wife, Alice Chen, said at the time.
Dr. Murthy will return as surgeon general at a critical moment, as the president tries to steer the nation out of the worst public health crisis in a century while expanding access to health care for millions of Americans. During his confirmation hearing, he told the Senate Health, Education, Labor and Pensions Committee that he would make ending the coronavirus pandemic his highest priority.
Dr. Murthy, 43, helped found Doctors for Obama, a group that worked to elect Mr. Obama and now works to expand health care access for Americans. It now goes by Doctors for America.
As an undergraduate at Harvard University, he helped found two nonprofits, one focusing on H.I.V./AIDS education in the United States and India, and another to train women as community health workers in rural India.
A son of Indian immigrants and the first person of Indian descent to hold the surgeon general’s post, Dr. Murthy, 43, was born in England and grew up mostly in Miami, watching his parents in their own medical practice. He invoked them during his confirmation hearing.
“I have tried,” Dr. Murthy said, “to live by the lessons they embodied: that we have an obligation to help each other whenever we can, to alleviate suffering wherever we find it, and to give back to this country that made their lives and my life and the lives of my children possible.”

Cineworld, the parent company of the U.S. movie theater chain Regal Cinemas, announced on Tuesday that it would reopen its cinemas in the United States in April and in Britain in May as those countries ease lockdown restrictions.
“We have long awaited this moment,” said Mooky Greidinger, the chief executive of Cineworld, which is based in London. “With capacity restrictions expanding to 50 percent or more across most U.S. states, we will be able to operate profitably in our biggest markets.”
Regal Cinemas is the second largest theater chain in the United States, after AMC Theaters. The announcement by Cineworld comes six months after the movie theater chains were forced to shut down across the United States and Britain last October in an effort to curb the spread of the coronavirus. The decision affected a total of 45,000 employees in both countries and forced studios to postpone film releases.
Cineworld also announced a multiyear agreement with Warner Bros. starting in 2022 that will allow the chain to show the studios’ films for 45 days in the United States and 31 days in Britain. The deal shortens the typical window that theaters have to show movies before they are released to on-demand streaming services.
The reopening plans in the United States will coincide with the release of two movies from Warner Bros. Pictures, “Godzilla vs. Kong” on April 2 and “Mortal Kombat” on April 16.
“We are very happy for the agreement with Warner Bros.,” Mr. Greidinger said. “This agreement shows the studio’s commitment to the theatrical business.”
Last week, AMC Theaters announced the reopening of nearly all of its U.S. theaters.
The moves come at a time of concern that looser restrictions will lead to a rise in coronavirus cases. On Monday, the director of the Centers for Disease Control and Prevention warned that relaxed pandemic restrictions could lead to another spike. “If we don’t take the right actions now,” said Dr. Rochelle Walensky, “we will have another avoidable surge.”
In September, Cineworld reported a pretax loss of $1.6 billion for the first half of 2020. In 2019, 90 percent of the company’s revenue was generated in the United States and Britain.

In New York City, nightclubs are still officially closed, and private gatherings have been limited to a maximum of 10 people since November. But finding a dance party is fairly simple once you know where to look.
First, you need to know who to follow on social media, where organizers and D.J.s post mildly cryptic fliers for events a couple of days in advance. Reach out via direct message, and you’ll get more detailed information.
Some parties are brazenly listed on Facebook, Instagram, Eventbrite and, until it was recently shut down, the party app Vybe Together. To avoid being caught, social media accounts are regularly wiped or lie dormant before reappearing to announce events.
A party’s location is usually given only the day of, and it could be anywhere: a private loft, a warehouse, a basement, a vacant office, even on a boat or a bus. Although many events are held in Manhattan and Brooklyn, an increasing number have moved to New Jersey, where indoor-gathering rules and enforcement are more lax.
In the rave scene, where the best parties have always been off the grid and on the down-low, attending an illegal event during a pandemic doesn’t seem so radical. But while many ravers are comfortable with a level of illegality, the pandemic tested others’ tolerance.
“Underground parties are not new in New York City,” said Sheriff Joseph Fucito, who, with his 150 deputies, has led the New York Police Department’s enforcement of virus restrictions. During the pandemic, the Sheriff’s Office has prioritized policing large events with multiple health or public safety hazards; the penalties are usually no greater than misdemeanor tickets to D.J.s, venue owners and, occasionally, attendees.