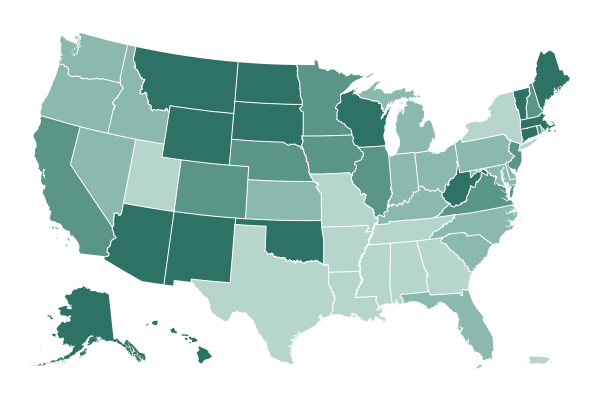
California surpassed 50,000 known coronavirus deaths on Wednesday, the first state to reach that chilling milestone.
The news comes as a bleak reminder that the recent progress the state has made against the pandemic may be fragile. Most of those deaths were recorded recently, during a frightening winter surge that followed a period of relatively low case counts and a spreading hope that the virus could be controlled until vaccines arrived.
According to a New York Times database, California, the country’s most populous state, averaged more than 560 deaths a day at its peak in January. By contrast, for much of November, it reported fewer than 50 deaths a day on average.
It took nearly 10 months for Los Angeles County to hit 400,000 cases, but little more than a month to add another 400,000, from Nov. 30 to Jan. 2.
Though the state has reported more total deaths than any other in the nation, it is far from the hardest hit relative to the size of its population. At least 30 states have reported more total deaths per capita, and New Jersey has recorded twice as many.
Tallying the loss of life across California’s vast expanse belies the virus’s uneven impact on poorer communities of color, particularly in the Central Valley and Los Angeles.
Latinos, who are more likely than other Californians to work in essential industries and less likely to have the resources or space to isolate themselves if they get infected, have been sickened and have died at disproportionately high rates. State figures show that Latinos, who make up 39 percent of the state population, accounted for 46 percent of California’s deaths.
“We’ve created a separate and unequal hospital system and a separate and unequal funding system for low-income communities,” said Dr. Elaine Batchlor, chief executive of Martin Luther King Jr. Community Hospital in Los Angeles, the hardest-hit hospital for its size in the hardest-hit county in the state.
And so far, California has failed to prevent the same inequities from plaguing the state’s vaccination effort, a process that has been criticized as chaotic and confusing.
In mid-November as Thanksgiving neared, state officials warned that another surge could be on its way. As cases rose again, leaders begged Californians to hunker down, and not to ease up on precautions. When they reimposed restrictions that had been lifted, the move added to a pervasive sense of exhaustion — another disheartening reversal in the pandemic.
Nearly all of California’s roughly 40 million residents spent the holidays under strict orders to stay at home. Gatherings with people they did not live with were banned.
Even with those restrictions, though, the virus spread rapidly and hospitals were overwhelmed.
Scenes like those that played out in New York during the spring — when testing was scarce and deaths were probably undercounted — became commonplace in Southern California, dashing experts’ hopes that they could be avoided.
The region was a center of the pandemic in the United States, just as the first vaccines were beginning to be administered.
Doctors and nurses treated patients in hospital lobbies. Relatives watched remotely as loved ones took their last breaths. Health care workers who held the screens for them are still grappling with the lingering effects of sustained trauma.
“It’s really hard to put all of it into words,” said Helen Cordova, an intensive care unit nurse at Kaiser Permanente Los Angeles Medical Center, the first person in California to get a vaccine shot outside a clinical trial.
On top of everything, researchers have confirmed that a coronavirus variant now spreading in California is more contagious than earlier versions of the virus.
Nevertheless, there is hope.
California is now reporting half as many new cases a day, on average, as it did two weeks ago. Some counties have been allowed to lift restrictions. Local officials say more reopenings are on the way. State lawmakers approved a $7.6 billion relief package this week.
And as Gov. Gavin Newsom — whose political fortunes hinge on getting children back into schools and shots into the arms of a far-flung, diverse populace — has pointed out, California has administered many more vaccine doses than any other state.

A new form of the coronavirus is spreading rapidly in New York City, and it carries a worrisome mutation that may weaken the effectiveness of vaccines, two teams of researchers have found.
The new variant, called B.1.526, first appeared in samples collected in the city in November. By the middle of this month, it accounted for about one in four viral sequences appearing in a database shared by scientists.
One study of the new variant, led by a group at Caltech, was posted online on Tuesday. The other, by researchers at Columbia University, has been submitted to a preprint server but is not yet public.
Neither study has undergone peer review or has been published in a scientific journal.
“It’s not particularly happy news,” said Michel Nussenzweig, an immunologist at Rockefeller University who was not involved in the new research. “But just knowing about it is good because then we can perhaps do something about it.”
Dr. Nussenzweig said he was more worried about the variant in New York than the one quickly spreading in California. Yet another contagious new variant, discovered in Britain, now accounts for about 2,000 cases in 45 states. It is expected to become the most prevalent form of the coronavirus in the United States by the end of March.
Patients infected with virus carrying that mutation were about six years older on average and more likely to have been hospitalized. While the majority of patients were found in neighborhoods close to the hospital — particularly Washington Heights and Inwood — there were several other cases scattered throughout the metropolitan area, said Dr. David Ho, director of the Aaron Diamond AIDS Research Center.
“We see cases in Westchester, in the Bronx and Queens, the lower part of Manhattan and in Brooklyn,” Dr. Ho said. “So it seems to be widespread. It’s not a single outbreak.”
Still, some experts were optimistic about the fight to control the spread of the disease, now that a number of vaccines are being distributed.
As the virus continues to evolve, the vaccines will need to be tweaked, “but in the scheme of things, those aren’t huge worries compared to not having a vaccine,” said Andrew Read, an evolutionary microbiologist at Penn State University. “I’d say the glass is three-quarters full, compared to where we were last year.”

Contagion and death have been intertwined with nursing homes since the coronavirus made its first appearance in the United States.
Some the grimmest chapters in the book of death the pandemic has written over the past year have been set in the very places where the weakest Americans were meant to be sheltered.
The virus has raced through some 31,000 long-term care facilities, killing more than 163,000 residents and employees. They accounting for more than a third of all virus deaths since the late spring.
But something is changing.
Our graphics team has taken a look at nursing home deaths and found heartening news.
Since the arrival of vaccines, which were prioritized to long-term care facilities starting in late December, new cases and deaths in nursing homes have fallen steeply, outpacing national declines, according to a New York Times analysis of federal data.
The turnaround is an encouraging sign for vaccine effectiveness and offers an early glimpse at what may be in store for the rest of the country, as more and more people get vaccinated.

With much of Europe living under heightened restrictions on movement and social interaction, the rates of Covid-19 infection across the continent have been cut in half from the winter peak, the World Health Organization said on Thursday.
But as pressure on national governments mounts to ease lockdowns, Hans Kluge, the W.H.O.’s director in Europe, cautioned new cases were still 10 times as high as they were last May and that the region was still experiencing high rates of community transmission.
“No one can predict the course of the pandemic,” Mr. Kluge said. “This really depends on our individual and collective measures.”
His caution reflected the broad concern over new virus variants while the infection rate remains stubbornly high.
Europe has now experienced close to 38 million coronavirus infection and at least 850,000 deaths. In the past two weeks, new cases have fallen below one million in the 53 countries covered by the W.H.O.’s European regional office.
But Europe has an increasing geographic spread of new infections and increasing prevalence of variants of concern, Catherine Smallwood, W.H.O. Europe’s senior emergency officer, told reporters.
More infections in the human population, means more variants will arise over time, she noted.
Public health officials have been pushing back at growing calls to open up economies and loosen controls as health services complete vaccinations of older and more vulnerable members of society — a campaign that has gotten off to a sluggish start in many nations.
“What we should be absolutely clear about is that will simply encourage the emergence of more dangerous variants,” said Martin McKee, professor of European public health at London University’s School of Hygiene and Tropical Health. “The places the variants have come from are the places with high levels of community transmission.”
European countries needed to step up their capacity for the genome sequencing used to detect characteristics of the virus and which enables scientists to spot the emergence of new variants. Only a small number of European countries are doing it, Mr. McKee said.
“That is a really high priority now,” he said.
As concerns grow that new coronavirus variants could blunt the protective effects of vaccines, Pfizer and BioNTech said on Thursday that they planned to test a third booster shot as well as update their original vaccine.
Laboratory experiments have found that the levels of antibodies neutralized by the Pfizer-BioNTech shot was reduced against a variant first identified in South Africa, which could hint at reduced efficacy. But there is no evidence yet from clinical trials there suggesting that the vaccine does not offer strong protection.
“We are taking multiple steps to act decisively and be ready in case a strain becomes resistant to the protection afforded by the vaccine,” Dr. Albert Bourla, the chief executive of Pfizer, said in a statement.
One study will look at what kind of protection is given when people receive a third shot about six to 12 months after the initial two-dose regimen. In addition, the companies said they were speaking to regulators about testing an adapted version of the vaccine that would protect against the variant from South Africa, known as B.1.351.
Moderna, which developed a vaccine using the same technology as the Pfizer-BioNTech product, said on Wednesday that it had shipped doses of a newly adjusted vaccine to the National Institutes of Health for testing. The adapted vaccine also addresses the B.1.351 variant, which seems to dampen the effectiveness of the existing vaccines.
Dr. Phil R. Dormitzer, Pfizer’s vice president and chief scientific officer of viral vaccines, said in an interview that the companies believed that a third shot, even in the original formulation, could strengthen the body’s immune system in fending off virus mutations.
The companies’ announcements come the same week that the Food and Drug Administration released draft guidance to the drug industry about adapting vaccines to address new variants. The agency said that companies would not have to undergo the same large-scale clinical trials that led to the original vaccines.
Dr. Gregory Poland, the director of the Vaccine Research Group at the Mayo Clinic in Rochester, Minn., said that Pfizer and other companies were smart to prepare for the possibility that a variant might make their vaccines less effective. He added that more information on how the original vaccines work against new variants was needed before fully switching gears.
“A variant can change in days and a completely different one can take over,” he said. “You’ve got to have good epidemiological evidence and good real-world effectiveness data to know is a variant vaccine worthwhile, and in whom.”
Because Pfizer and BioNTech believe their existing vaccine still offers good protection against the variants, Dr. Dormitzer said, their adapted vaccine plan is more like a test run — a way of learning how to quickly create and study a tweaked vaccine in case a more threatening mutation develops in the future.
“The thought behind the test is not that we think we need to change the vaccine right now — we think we probably do not,” Dr. Dormitzer said.
But, he said, the companies could apply lessons they learn from the clinical trials they are planning “if we find ourselves in a situation in the future where we really had to move fast because a new strain started circulating that was not covered by the vaccine.”

The hope brought by the arrival of the first vaccines in South America is hardening into anger as inoculation campaigns have spiraled into scandal, cronyism and corruption, rocking national governments and sapping trust in the political establishment.
Four ministers in Peru, Argentina and Ecuador have resigned this month or are being investigated on suspicion of receiving or providing preferential access to scarce coronavirus shots. Prosecutors in those countries, and in Brazil, are examining thousands more accusations of irregularities in inoculation drives, most of them involving local politicians and their families cutting in line.
As accusations of wrongdoing ensnare more dignitaries, tension is building in a region where popular outrage with graft and inequality have spilled in recent years into raucous protests against the political status quo. The frustration could find an outlet in the streets again — or at the polls, shaping voter decisions in Peru’s elections in April and other upcoming races.
“They all knew that patients have been dying,” Robert Campos, 67, a doctor in Lima, Peru, said of the country’s politicians. “And they vaccinated all their little friends.”
The anger at powerful line cutters has been amplified by the scarcity of the vaccines. South America, like other developing regions, has struggled to procure enough doses as rich nations bought up most of the available supply.
Dr. Campos said he did not make the vaccination list when limited doses arrived for hospital staff last week.
South America was shattered by the virus, accounting for nearly a fifth of all pandemic deaths worldwide — 450,000, according to the official tally — despite representing about 5 percent of the world’s population. Mortality data suggests the pandemic’s real toll on the region is at least double the official numbers.
The virus also collapsed national health care systems, pushed millions into poverty and plunged the region into its worst economic crisis in modern history.
Despite the heavy toll, the pandemic shored up public support for most of the region’s governments as several offered financial support to their populations and called for unity.
The vaccine scandals could bring this good will to an end, heralding a new wave of instability, analysts warn.
“People find it much more difficult to tolerate corruption when health is at stake,” said Mariel Fornoni, a pollster in Buenos Aires.
Mitra Taj, Anatoly Kurmanaev, Manuela Andreoni and

The two-dose Covid-19 vaccine developed by Pfizer and BioNTech is protecting recipients about as well in wide actual use as it did in clinical trials, according to a new large-scale study from Israel that was published on Wednesday in The New England Journal of Medicine.
The study, by the Clalit Research Institute, the research arm of Israel’s largest H.M.O., in collaboration with experts from Harvard University and Boston Children’s Hospital, found that the vaccine reduced symptomatic cases by 94 percent a week after the second dose, and reduced severe disease by 92 percent.
The study appears to be the first large-scale, peer-reviewed examination of the vaccine’s performance in general use. It included more than a million people aged 16 and over, nearly 600,000 of whom had been vaccinated, and an equally large, carefully matched control group of unvaccinated individuals.
The results reflect those of earlier studies out of Israel, as well as clinical trials showing a 95 percent efficacy rate for the vaccine.
“You’re never quite sure, after a controlled trial, will it really look like this in the real world?” Dr. Phil R. Dormitzer, vice president and chief scientific officer of viral vaccines at Pfizer, said in an interview. “So that’s some good news.”
Israel’s swift inoculation campaign has outpaced the rest of the world, making the country a kind of test laboratory for the two-dose Pfizer-BioNTech vaccine. More than half the nation’s nine million people have received the first dose, and more than one-third have received both.
The country has universal health care, and about 53 percent of the population is enrolled in Clalit Health Services, giving researchers access to a huge pool of data that could be used to make certain that they were drawing sound conclusions.
“In all studies of vaccine effectiveness, a major challenge is to ensure that those we are comparing to identify the vaccine’s effect are similar in the other characteristics that may predict whether they get infected or ill,” said Prof. Marc Lipsitch of the Harvard T.H. Chan School of Public Health, who took part in the study. “Clalit’s extraordinary database made it possible to design a study that addressed these challenges.”
“We have more than 20 years of fully digitized electronic medical records,” said Prof. Ran Balicer, who directs Clalit’s research institute and is the senior author of the new study.
The study included some 22,000 vaccine recipients aged 80 or above, a much larger sample of this exceedingly high-risk category than Pfizer had in its randomized clinical trials. The new study found no drop in effectiveness for the vaccine among older people.
“This research is a perfect example of how randomized trials and observational health care databases complement each other,” Prof. Miguel Hernán, another Harvard researcher who took part in the study, said in a statement.
The study began when Israel started its vaccination drive on Dec. 20, and continued until Feb. 1 — a period when Israel was going through its third and largest wave of infection, and when the B.1.1.7 variant, first detected in Britain, was becoming the dominant source of new cases in Israel. The study indicated that the vaccine was effective against that variant.
Katie Thomas contributed reporting.

European leaders, deeply concerned that another summer’s lucrative tourism trade could be lost to the pandemic, are escalating calls for the European Union to introduce a common system that would allow borders to reopen to people who have been inoculated against the virus.
Even as Europe’s vaccination program contends with long delays and one senior European Union official admitting it would be “difficult” to reach the bloc’s goal of vaccinating 70 percent of adults by the end of summer, the idea of a European Union-wide vaccine passport system has become a hotly discussed topic.
Senior officials in Greece and Spain — countries heavily reliant on tourism — are among those who have supported proposals for a program of so-called vaccine passports.
They argue that requiring people to show a certificate proving they have received a coronavirus shot would restore the bloc’s pillar of free movement, help draw in summer holidaymakers and allow business trips to return.
Before a scheduled online meeting on Thursday of the heads of all 27 European Union nations, the Austrian chancellor, Sebastian Kurz, added his voice in support of the idea.
“We want to get back to normal as quickly as possible, have our old lives back and maximum freedom,” Mr. Kurz said in a tweet on Wednesday. “We therefore want an EU-wide Green Passport, with which people can travel freely, do business without restrictions and go on holiday, as well as finally enjoy gastronomy, culture, events and other things again.”
But there is concern brewing that introducing a vaccine passport system so early in Europe’s vaccination program would create a two-tier system by the summer of inoculated people who could travel carefree while those yet to be vaccinated would be grounded.
European leaders are not expected to make a decision at the summit meeting on Thursday on the use of vaccination certificates, but they are expected to discuss how to ensure such a program would be able to run across all countries in the bloc.
In an interview with Bild Live, a digital offshoot of the German tabloid, Mr. Kurz urged that the passport system to be one easily accessible on a cellphone.
He said he supported the idea “so everyone can have all the freedoms back that we value so much,” adding that he was “personally very optimistic about the summer.”

With many businesses in Poland in open revolt against coronavirus restrictions — and their cause increasingly backed by the court — the Polish government is hoping to blunt a recent rise in cases by turning to the one tool known to work: masks.
The Polish government announced this week that face coverings like scarves and bandannas can no longer be worn instead of protective masks.
The announcement came as the number of new cases rose for a second straight day — with some 12,000 new infections detected on Wednesday.
“The third wave of the epidemic is gaining momentum,” said the Polish health minister, Adam Niedzielski, during a news conference on Wednesday.
He also said that restrictions would be tightened in the Northeastern region of the country where the growth in cases has been highest.
Children in early primary school will be forced to return to remote learning and galleries, museums, swimming pools, movie theaters and hotels will have to close down again — less than two weeks after they were allowed to reopen.
The minister, who is currently self-isolating after coming into contact with a virus-infected member of the government during a news conference last Friday, announced additional restrictions on the Polish southern border with Slovakia and the Czech Republic.
Both of those neighboring countries have seen even larger surges in new cases and nearly all people entering Poland from those nations will have to present proof of a negative coronavirus test or proof of complete vaccination.

The drugmaker Moderna has created a new, experimental form of its coronavirus vaccine to combat a worrisome variant of the virus, and has also begun to increase its overall manufacturing capacity, the company announced on Wednesday.
The new version of the vaccine, directed against a variant first identified in South Africa and now found in the United States and dozens of other countries, has been sent to the National Institutes of Health for testing. Studies have suggested that vaccines may be less effective against this variant than against the form that emerged earlier in the pandemic.
But the new version is probably months away from public use. The company outlined several possible approaches for evaluating the experimental form. Initial test results may be available by summer, Dr. Tal Zaks, Moderna’s chief medical officer, said in an interview.
Moderna’s vaccine uses genetic material called mRNA, a technology that allows researchers to create and adapt vaccines much faster than traditional methods. The Pfizer-BioNTech vaccine is also based on mRNA, and Dr. Ugur Sahin, the chief executive of BioNTech, said last month that the company could produce a new version within about six weeks if necessary.
The Food and Drug Administration said on Monday that new versions of existing vaccines, adapted to target variants of the coronavirus, would not have to go through the same large trials in 30,000 or 40,000 patients as the first vaccines did.
Moderna said that its original vaccine still provides some protection against the variant, but that “out of an abundance of caution,” the company would pursue several new approaches.
One plan would use a shot of the new vaccine as a booster, after two doses of the original formulation.
Another strategy would be a booster shot combining the new vaccine and the original one.
A third approach, already underway, involves giving a third shot of just the original vaccine as a booster.
These booster shots would use a smaller dose of vaccine, 50 micrograms, instead of the 100 micrograms given in each of the shots now being administered in a two-dose series.
The company said it also planned to evaluate full, 100-microgram doses of the new vaccine and the combination shot as primary vaccinations, rather than merely boosters.
The company also said it was making new capital investments that it expected would enable it to manufacture 1.4 billion doses of coronavirus vaccine in 2022. That figure assumes that a dose is 100 micrograms, but if research finds that smaller amounts are effective, the number of doses would be even higher.
Moderna also said that its production plan for 2021 had increased, to 700 million doses from 600 million, and that it was trying to “potentially deliver up to 1 billion doses in fiscal year 2021.”
The company said it had already shipped 60 million doses globally, including about 55 million to the U.S. government, and expected to complete delivery of the first 100 million U.S. doses by the end of the first quarter of 2021, the second 100 million by the end of May and the third 100 million by the end of July.

Long before Orrin Heatlie filed recall papers, he knew the odds were against unseating Gavin Newsom, the suave ex-mayor of San Francisco who had ascended to become California’s governor.
“Democrats have a supermajority here; it’s one-party rule,” said Mr. Heatlie, a Republican and retired Yolo County sheriff’s sergeant. Voters elected Mr. Newsom in 2018 by a record 24-point margin. As recently as April, he had a 70 percent approval rating. Mr. Heatlie’s recall petition requires about 1.5 million valid voter signatures just to trigger a vote.
Lately, however, he has been feeling lucky.
The coronavirus has upended California. Most of the state is waiting for vaccinations. Schools in big cities have yet to reopen. As much as $30 billion has been looted from the state’s pandemic unemployment insurance program.
And then there was that dinner the governor attended, barefaced, after telling Californians to stay in and wear masks.
“This is an easy sell,” Mr. Heatlie reported last week, saying he had exceeded 1.7 million signatures three weeks before the deadline.
Mr. Newsom is one of many chief executives across the country to become a magnet for the rage and grief of pandemic-weary Americans.
In Ohio, Gov. Mike DeWine, has been assailed for strict enforcement of health precautions. Gov. Greg Abbott of Texas was under fire for runaway infection rates in border cities. Crashes of the vaccine system in Massachusetts have eroded the popularity of Gov. Charlie Baker.
And in New York, Gov. Andrew M. Cuomo’s image as a national leader during the pandemic has suffered over New York’s counting of coronavirus deaths of nursing home residents.
Dane Strother, a Democratic media consultant in California who represents officials across the country, said governors “are in an untenable position.”
As California works the kinks out of its vaccine rollout and starts to reopen classrooms, it is tough to determine whether recall efforts will succeed. If the recall petitions qualify, voters would be asked two questions: Should Mr. Newsom be recalled, and if so, who should complete his term.
For now, fellow Democrats have closed ranks around Mr. Newsom, and the White House press secretary, Jen Psaki, emphasized this month that President Biden “clearly opposes any effort” to recall the governor.
When reporters recently asked about the recall effort, the governor said, “I’m focused on the vaccine issue.” His team, however, notes that recall attempts are not unusual in California: recall petitions have been filed against every governor in the last 61 years.
Already three Republicans — Kevin Faulconer, the former mayor of San Diego; the conservative activist Mike Cernovich; and John Cox, who lost to Mr. Newsom in 2018 — say they would challenge the governor, and Richard Grenell, acting intelligence chief under former President Donald Trump, would not rule it out.
The recall effort has also has tapped into a bipartisan unease as the virus’s death toll in California reached 50,000 lives on Wednesday.
In California, Republican registration has been falling for years. The party now represents less than a quarter of registered voters, but as Mr. Newsom has awkwardly constrained 40 million Californians in the name of safety, Republicans have sought to energize their base.
Harmeet Dhillon, a Republican national committeewoman and San Francisco lawyer, has peppered Mr. Newsom with pandemic-related lawsuits, filing on behalf of churches, and gun shops. Far-right groups have rallied against masks and business closures, and conservative sheriffs have refused to enforce state health rules.
Mr. Heatlie and the coalition sued to extend the Nov. 17 deadline and got four more months in a court decision on Nov. 6.
That evening, Mr. Newsom and his wife were photographed at the exclusive French Laundry restaurant at a birthday dinner for a lobbyist friend.
At that point, only 55,588 people had signed Mr. Heatlie’s petitions. One month later, he had nearly half a million signatures.
Winning in deep-blue California, however, will not be easy.
“Newsom came into office dealing with wildfires and spent the past year trying to handle a pandemic — he’s basically trying to govern in the Book of Revelation,” said David Townsend, a Democratic consultant who specializes in ballot measures. “I think voters will see that.”

Two Chinese vaccine makers have said that their Covid-19 vaccines are effective at preventing serious illness, paving the way for their deployment in China and the developing world over the next few months.
The news indicates that China is likely to have four vaccines approved for general use — two vaccines are already being mass produced, by Sinopharm and Sinovac Biotech. The addition of two more could significantly speed up China’s mass inoculation drive, which has been slow in part because the government is prioritizing the export of its vaccines.
All the vaccines have been shown to prevent severe illness. But they have been dogged by a lack of transparency around clinical data.
CanSinoBIO, which has teamed up with a military institute that belongs to the People’s Liberation Army, said on Wednesday that its one-shot vaccine had an efficacy rate of 65.28 percent at preventing all symptomatic cases. Separately, Sinopharm, which has a vaccine that is already in use in China, said the shot that it developed with its affiliate, the Wuhan Institute of Biological Products, had an efficacy rate of 72.51 percent against Covid-19.
Both companies said they had asked Chinese regulators for approval for public use. They gave few details on their analyses, such as how many people contracted Covid-19 during the trials, giving scientists little data to make independent assessments.
Several developing countries have already ordered the vaccines, which can be easily stored at refrigerated temperatures.
Like other Chinese vaccine makers, CanSino had to go abroad to start its Phase 3 clinical trials, testing its vaccine in five countries: Argentina, Chile, Mexico Pakistan and Russia. The company’s vaccine has already been approved for use by the military.
Unlike Sinopharm and Sinovac, CanSino’s chief executive, Yu Xuefeng, has indicated that the company could struggle to ramp up production to meet the needs of China’s 1.4 billion people. Mr. Yu has said previously that the company’s vaccine production capacity was 100 million doses per year, or 200 million doses at the most.
The CanSino vaccine is made with a virus, called Ad5, that is modified to carry genetic instructions into a human cell. The cell begins making a coronavirus protein and the immune system learns to attack it. Before the release of the efficacy data, scientists were doubtful that the Ad5 vector would work effectively because it is a cold virus that many people are likely to have been exposed to.
Sinopharm tested its Wuhan vaccine in seven countries, including Bahrain, Egypt and the United Arab Emirates. In December, the vaccine that it developed with the Beijing Institute of Biological Production was approved for use. Like the Beijing vaccine, the Wuhan shot was made using a tried-and-tested technology that relies on a weakened virus to stimulate the immune system.
Both Sinopharm vaccines were approved in July for emergency use and rolled out to thousands of health care workers and travelers even before the completion of Phase 3 trials. The company said it could produce a maximum of one billion doses this year.

The list of announcements at the Maranatha Baptist Church in Plains, Ga., on Sunday included some routine business. There was a reminder of a deacons’ meeting immediately following the service and a request for donations of macaroni and cheese for a local food bank.
Then the pastor said he had one additional announcement to share, and it was good news: Jimmy and Rosalynn Carter were back.
The former president, 96, and his wife, 93, had returned to the church to worship in person for the second Sunday in a row, now that both had received vaccinations against the coronavirus, the pastor, Tony Lowden, said.
“Let’s welcome them back,” Pastor Lowden told the congregation, according to a video of the service posted on the church’s Facebook page. The Carters, wearing masks, waved from their familiar spot in the front pew, acknowledging applause from the church.
Pastor Lowden gently reminded the members that if they “get tackled” by the Secret Service when approaching the Carters, it would only be because the church was practicing social distancing.
The Carters have long been devoted members of Maranatha Baptist — she as a deacon, and he as a deacon and, for many years, a Sunday school teacher.
The Sunday school classes, which he no longer teaches, for decades drew Democratic presidential candidates and visitors from across the country, who made pilgrimages to hear the former president teach at the church in the tiny southwest Georgia farming community where he was raised.